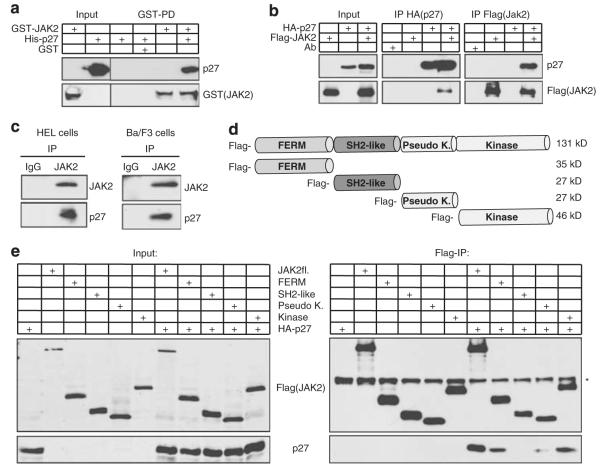
Figure 3.
JAK2 directly interacts with p27Kip1 through its FERM and kinase domain. (a) GST and GST-JAK2 fusion proteins isolated from E.coli were bound to glutathione sepharose and incubated with recombinant purified His-p27Kip1. After extensive washing, GST-JAK2-associated p27Kip1 was detected in immunoblots. (b) p27Kip1 interacts with JAK2 in 293T cells. HA-p27Kip1 and Flag-JAK2 were overexpressed and expression was verified by immunoblotting. For the co-preciptiation analysis, HA-p27Kip1 or Flag-JAK2 were immunoprecipitated using tag-specific antibodies. Immunoprecipitated proteins were detected in immunoblots. (c) Endogenous p27Kip1 co-immunoprecipitates with endogenous JAK2 and JAK2V617F in hematopoietic cells. JAK2V617F was immunoprecipitated from HEL cells and JAK2 from IL-3-starved (16 h) Ba/F3 cells and analyzed for co-immunoprecipitated p27Kip1 in immunoblots. (d) Schematic representation of JAK2 domains that are used in (e). p27Kip1 interacts with JAK2, its isolated FERM- and kinase domains. Flag-tagged JAK2 and Flag-tagged JAK2 domains were expressed with HA-p27Kip1 in 293T cells. Their expression was confirmed in immunoblots ((e), left panel, ‘Input’). Flag-tagged proteins were immunoprecipitated with Flag-antibodies and precipitates were analyzed in immunoblots ((e), right panel, ‘Flag-IP’). p27Kip1 co-immunoprecipitates with JAK2, its FERM domain and its kinase domain. A weak interaction could be observed with the pseudokinase domain. The asterisk (*) indicates heavy chain.