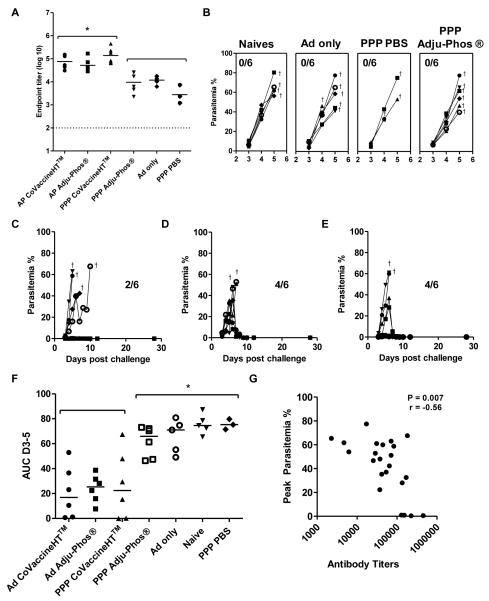
Figure 7. An adenoviral prime improves efficacy of MSP-1 protein vaccines following P. yoelii blood-stage challenge.
BALB/c mice (n= 3-6 / group) were immunized i.m. with either i) 1.5 μg of P. yoelii MSP-119-GSTprotein in Adju-Phos® or CoVaccine HT™or PBS three weeks apart (PPP); or ii) primed with 1×1010 vp of AdHu5 MSP-142 and either not boosted (Ad only) or boosted eight weeks later with 1.5 μg of P. yoelii MSP-1-GSTprotein in Adju-Phos® or CoVaccine HT™ 19 (AP). IgG titers were measured in the serum in response to (A) P. yoelii MSP-119-IMX108 protein two weeks after the final immunization (day before challenge). * p<0.05 by one-way ANOVA with Bonferroni’s multiple comparison post-test. Median responses are shown. Mice were challenged with 105 pRBCs i.v. and parasitemia was measured as the percentage of infected red blood cells over time. Results are shown in (B) for the naïve unimmunized, Ad only and PPP PBS control groups as well as the PPP Adju-Phos® group; (C) mice immunized with PPP in CoVaccine HT™; (D) mice immunized with AP Adju-Phos®; and (E) mice immunized with AP CoVaccine HT™. Crosses indicate when mice were sacrificed. (F) AUC analysis of parasitemia. * p<0.05 by one-way ANOVA with Bonferroni’s multiple comparison post-test. Median responses are shown. (G) IgG titers measured two weeks after the final immunization in each regime were correlated with percentage peak parasitemia. Spearman’s rank correlation is shown. The dotted line indicates the threshold for responses above background in (A).