Abstract
This is the first investigation of the relationship between parental age and extreme social-communicative autistic traits in the general population. The parents of 5,246 children in the Avon Longitudinal Study of Parents and Children (ALSPAC) completed the Social and Communication Disorders Checklist (SCDC). The association between parental age and SCDC scores was assessed in the full sample and among high scoring individuals (e.g. top 5%, 1%). There was no association between parental age and social-communicative autistic traits in the general population. Neither maternal nor paternal age was associated with extreme scores. These findings suggest that advanced parental age does not confer increased risk for extreme social and communication impairment assessed quantitatively.
Keywords: Autism spectrum disorders, Autistic traits, arental age, ALSPAC
Introduction
The association between parental age and autism spectrum disorders (ASD) has been investigated extensively, with conflicting results (e.g. Cantor et al. 2007; Croen et al.2007; Durkin et al. 2008; Grether et al. 2009; King et al. 2009; Olsen and Zhu 2009; Sasanfar et al. 2010; Shelton et al. 2010; Williams et al. 2008). Only one previous study, however, has investigated the relationship between parental age and autism-like behaviors in the general population (Lundstrom et al. 2010). Using two nationally-representative twin samples, Lundstrom et al. (2010) reported higher than average autistic trait scores among children whose fathers were younger than 25 or older than 40.
This study is the first to investigate: (a) the relationship between maternal age and social-communicative autistic traits in the general population and (b) the association between parental age and extreme social and communication impairment assessed quantitatively. The size of this study (n = 5,246) permits investigation of the top 1% of the trait distribution, a group whose prevalence and mean parent-rated trait scores mimic those estimated for ASD (Robinson et al. 2011). Moreover, examining the association between parental age and autism-like behaviors in population based non-clinic referred samples is important as selection factors, including comorbidity, influence treatment seeking (Cohen and Cohen 1984). General population high scorers constitute a representative sample of individuals with a high burden of parent-rated autistic traits.
Methods
Sample
This study utilizes a subsample of the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, a nationally representative sample in the United Kingdom. ALSPAC enrolled 14,541 pregnancies with expected delivery dates between April 1, 1991 and December 31, 1992 in Avon county, United Kingdom. All women in the study area with those due dates were eligible to participate. The initial ALSPAC cohort included the 13,988 children alive and in follow up at one year of age. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committee.
To analyze the potential mediating role of cognitive covariates, this analysis was restricted to children who completed an IQ assessment at age 8, during the ‘Focus at 8’ clinical assessments. Slightly more than half (n = 7,044, 50.4%) of the initial cohort completed the IQ test. The sample was further limited to singleton children whose parents completed the autistic traits measure (SCDC; Social and Communication Disorders Checklist) at age 7. The final sample (n = 5,246) also excluded individuals with missing data on paternal age (n = 363) and maternal education (n = 75). Children in the analyzed sample were 50.0% male, 96.7% white, and 46.5% of their mothers completed one or more A-level exams. In contrast to the full ALSPAC cohort, this subsample included fewer non-white children (full sample: 95.0%; χ2 = 25.19, df = 1, p < 0.001) and more children whose mothers had completed at least one A-level exam (full sample: 35.3%; χ2 = 193.73, df = 1, p < 0.001). The analyzed sample is reasonably representative of the UK as a whole. Employing results from the General Household Survey (Office for National Statistics, 2005), 92% of the population is white, 50% of children are male, and 32% of mothers have completed one or more A-levels.
Measures
Autistic Traits
Autistic traits were assessed using the Social and Communication Disorders Checklist (SCDC) (Skuse et al. 2005), a 12 item parent-report measure. Its questions are designed to assess social and communication skills over the past 6 months. Total scores range from 0 to 24 where 24 indicates the highest level of autistic traits. The SCDC displays high internal consistency (α = 0.93) and strong discriminant validity in the ALSPAC sample (Skuse et al. 2005). The SCDC does not assess restricted and repetitive behaviors and interests (RRBI), a definitional domain of ASD. Accordingly, this study assessed only the relationship between parental age and social-communicative autistic traits.
Parental Age
Both maternal and paternal age were reported by mothers. Maternal birth date was recorded at the time of study enrollment; maternal age at birth was derived from the difference between date of birth and date of delivery. Paternal age at birth was estimated from the question, “What is the age of your partner, in years?” included in a survey administered during the pregnancy. For the purposes of this analysis, parental age was grouped into integer values (e.g. 30, 31, 32).
Child IQ
Child IQ was assessed using an abbreviated version of the Weschler Intelligence Scale for Children-Third Edition (WISC-III), a commonly used scale designed to measure cognitive potential in children between the ages of 6 and 16 (Weschler et al. 1992).
Covariates
Covariates were selected based on prior evidence suggesting a relationship with both parental age and ASD (Fraser et al. 1995; Larsson et al. 2005; Peipert and Bracken 1993; Saha et al. 2009). Maternal psychiatric history was derived from four items, inquiring whether the respondent had ever experienced: (a) anorexia, (b) schizophrenia, (c) a major depressive episode, or (d) other major psychiatric disorder. As the reported prevalences of schizophrenia and anorexia were very low (0.13 and 1.79% respectively), a summary variable was created such that experience of any one of the four was coded as positive history for psychiatric disorder; no experience was coded as negative history. Substance abuse history was similarly treated as a combination of past self-reported experience with either alcohol or other drug addiction. Maternal educational achievement at pregnancy (e.g. vocational training, O-level or A-level exams, degree) and pregnancy complications (c-section delivery, infection during pregnancy) were assessed through self report.
Analysis
These analyses were designed to evaluate the relationship between parental age and social-communicative autistic traits in the general population. First, ANCOVAs (analysis of covariance) were employed to investigate whether parental age, coded in 5-year intervals, was associated with SCDC scores in the full sample. Second, we examined whether parental age varied across high scoring groups. High scorers were identified as those whose parent-rated trait scores fell within the top 1, 5, and 10% of the sample distribution. Extreme scoring categories were non-overlapping (e.g. 90–94%, 95–98%, ≥99%). Third, logistic regression models were estimated to examine whether parental age, treated continuously, was linearly or quadratically associated with trait scores above the a) 90th or b) 99th percentiles. Adjusted models controlled for coparent age, SES, gestation length, pregnancy complications, and maternal psychiatric history. Were significant associations between parental age and autistic traits found in any of the three sets of analyses, further models would have examined whether those relationships were mediated by child IQ.
Results
Descriptive Statistics
SCDC scores ranged between 0 and 23, with a mean of 2.69 (sd = 3.45). SCDC values were greater for males (mean = 3.09) than females (mean = 2.29; t = −8.41, df = 4921.8, p < 0.001). Mean SCDC scores were greater for individuals with low IQ (below 75; mean = 5.61) than those without (mean = 2.62; t = −6.90, df = 135.2, p < 0.001). Paternal age ranged from 15 to 65 (mean = 31.65, sd = 5.45); maternal age ranged from 15 to 44 (mean = 29.50, sd = 4.38). SCDC scores were negatively correlated with child IQ (r = −0.14, p < 0.001) but displayed no relationship with either maternal (r = −0.02, p = 0.10) or paternal (r = −0.01, p = 0.38) age. Controlling for paternal age and maternal education, there was a positive relationship between maternal age and child IQ. A 3-year increase in maternal age predicted a 1 point increase in full IQ score (p < 0.001). There was no relationship between paternal age and child IQ after adjusting for maternal age and education (p = 0.80).
Full Sample Analysis
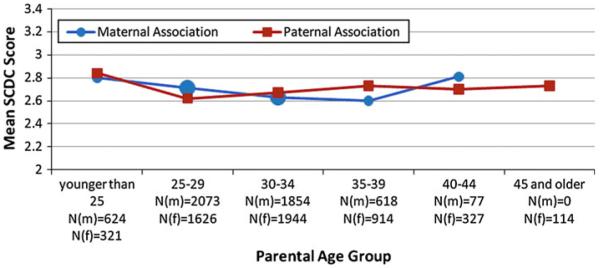
Results of the full sample analysis are presented in Fig. 1
Fig. 1.
Relationship between parental age and autistic traits in the general population. Note: Mean SCDC scores following adjustment for: co-parent age, maternal and paternal socioeconomic status, maternal psychiatric history, history of pregnancy complications N(m) = number of mothers in each age group; N(f) = number of fathers in each age group
There was no association between maternal age and social-communicative autistic trait scores in either unadjusted (F = 0.80, df = 4, p = 0.52) or adjusted (F = 0.31, df = 4, p = 0.87) analyses. Controlling for covariates, the mean SCDC scores associated with the maternal age groups (n = 5) ranged from 2.60 to 2.81. Similarly, there was no association between paternal age and social-communicative autistic trait scores in unadjusted (F = 0.57, df = 5, p = 0.73) or adjusted (F = 0.27, df = 5, p = 0.93) analyses. Controlling for covariates, the mean SCDC scores associated with the paternal age groups ranged from 2.62 to 2.84.
Parental Age and Extreme Scoring Groups
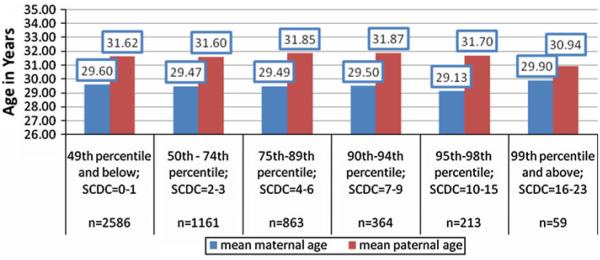
The comparison of parental age between extreme scoring groups and the general population is presented in Fig. 2
Fig. 2.
Mean parental age across the distribution of quantitative trait scores. Note: Mean parental age following adjustment for: co-parent age, maternal and paternal socioeconomic status, maternal psychiatric history, history of pregnancy complications
There was no association between maternal age and extreme group status in either unadjusted (F = 1.80, df = 5, p = 0.11) or adjusted (F = 1.14, df = 5, p = 0.34) ANOVA analyses. There was no association between paternal age and extreme group status in either unadjusted (F = 1.33, df = 5, p = 0.25) or adjusted (F = 1.00, df = 5, p = 0.42) ANOVA analyses. Maternal and paternal age were neither linearly (0.08 < p < 0.70) nor quadratically (0.15 < p < 0.98) associated with scores above the 90th or 99th percentiles in raw or adjusted logistic regression models (not tabled). As neither the full sample nor extreme group analyses suggested a main effect of parental age, mediation analyses were not warranted.
Discussion
These findings suggest that there is no association between parental age and social-communicative autistic traits in the general population, either in the full sample or at the extremes of the behavioral distribution. Extreme scorers falling within the top 1% show a similar prevalence and estimated symptom level to individuals with an ASD (Robinson et al. 2011). Accordingly, the null findings within the top 1% correspond to those of Williams et al. (2008), who reported no association between parental age and probability of ASD diagnosis in the ALSPAC sample. As the cases in both analyses (quantitative high scorers, individuals with an ASD diagnosis) were ascertained from the same population-based sample, these findings suggest consistency between the etiology of extreme autistic traits and qualitatively-defined ASD with regard to parental age.
The full sample findings contradict those of Lundstrom et al. (2010) who reported a significant, quadratic effect of paternal age on autistic traits in both UK and Swedish general population samples. This difference may be attributable to the measures used to assess autistic traits. The Lundstrom study employed the Childhood Autism Spectrum Test (CAST) (Scott et al. 2002; Williams et al. 2006) in the UK and A-TAC (Autism – Tics, AD/HD, and other Co-morbidities inventory) (Hansson et al. 2005) in Sweden. While both measures include RRBI items, this is unlikely to drive the difference in findings: the majority of the effect noted in both the UK and Swedish samples was attributable to the communication impairment domain.
Limitations
The primary limitation of this study is the measure of paternal age. Mothers were asked to identify the age of their ‘partner’, who was not explicitly defined as the biological father of the child. Accordingly, the estimate of paternal age could have been affected by both:( a) interpretation of the term partner and (b) error due to mothers’ miss-estimation of partner age. There is no reason to believe, however, that these factors would result in systematic over- or under-estimation of paternal age. Therefore, the measurement problem should be interpreted as one of reliability rather than validity. A reduction in reliability does not bias estimates; it will, however, attenuate statistical associations. Secondarily, these questions should be re-examined (a) using a measure that captures RRBI and (b) in a population in which parents in extreme age categories have been oversampled. Though there were no significant differences in mean child SCDC scores within the categories at the extremes (e.g. fathers aged 15–19 vs. fathers aged 20–24), this study was likely underpowered to consider that variation given the limited number of parents aged 45 and above and 19 and below. Lastly, these analyses depend exclusively on parent-rated social-communicative autistic trait scores. These questions should be reexamined in an analysis involving trained or multiple raters.
Conclusions
These findings suggest that there is no association between parental age and social-communicative autistic traits in the general population, either in the full sample or at the extremes. General population analyses may be highly useful in epidemiologic study of autism risk. Studies that assess factors related to a diagnosis of autism are subject to confounding by both: (a) variables that predict receiving a diagnosis of ASD and (b) variables that predict entry into a diagnostic sample. General population findings may help in understanding the degree to which those variables influence ASD risk profiles.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The authors specifically thank Drs. Jean Golding and Alan Emond for their helpful comments regarding this project. The UK Medical Research Council (74882), the Wellcome Trust (076467) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Elise Robinson, who will serve as guarantors for the content of this paper. The research was specifically funded by a National Institute of Mental Health/NIH Research Fellowship in Mental Health and Developmental Disabilities (MH/DD) at The Children’s Hospital Boston, Harvard Medical School (MH71286) and the Training Program in Psychiatric Genetics and Translational Research at the Harvard School of Public Health (T32MH017119).
Contributor Information
Elise B. Robinson, Department of Epidemiology, Harvard School of Public Health, Simches Research Building, 185 Cambridge Street, 6th floor, Boston, MA 02114, USA Psychiatric and Neurodevelopmental Genetics Unit, Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA, USA; Division of Developmental Medicine, Children’s Hospital Boston, Boston, MA, USA.
Kerim Munir, Division of Developmental Medicine, Children’s Hospital Boston, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
Marie C. McCormick, Department of Society, Human Development, and Health, Harvard School of Public Health, Boston, MA, USA
Karestan C. Koenen, Departments of Society, Human Development, and Health, and Epidemiology, Harvard School of Public Health, Boston, MA, USA Harvard Center on the Developing Child, Cambridge, MA, USA.
Susan L. Santangelo, Department of Epidemiology, Harvard School of Public Health, Simches Research Building, 185 Cambridge Street, 6th floor, Boston, MA 02114, USA Psychiatric and Neurodevelopmental Genetics Unit, Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
References
- Cantor RM, Yoon JL, Furr J, Lajonchere CM. Paternal age and autism are associated in a family-based sample. Molecular Psychiatry. 2007;12(5):419–421. doi: 10.1038/sj.mp.4001966. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J. The clinician’s illusion. Archives of General Psychiatry. 1984;41:1178–1182. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Archives of Pediatrics and Adolescent Medicine. 2007;161(4):334–340. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, et al. Advanced parental age and the risk of autism spectrum disorder. American Journal of Epidemiology. 2008;168(11):1268–1276. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. New England Journal of Medicine. 1995;332(17):1113–1117. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- Grether JK, Anderson MC, Croen LA, Smith D, Windham GC. Risk of autism and increasing maternal and paternal age in a large north American population. American Journal of Epidemiology. 2009;170(9):1118–1126. doi: 10.1093/aje/kwp247. [DOI] [PubMed] [Google Scholar]
- Hansson SL, Rojvall A. Svanstrom, Rastam M, Gillberg C, Anckarsater H. Psychiatric telephone interview with parents for screening of childhood autism - tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): preliminary reliability and validity. British Journal of Psychiatry. 2005;187:262–267. doi: 10.1192/bjp.187.3.262. [DOI] [PubMed] [Google Scholar]
- King MD, Fountain C, Dakhlallah D, Bearman PS. Estimated autism risk and older reproductive age. American Journal of Public Health. 2009;99(9):1673–1679. doi: 10.2105/AJPH.2008.149021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161(10):916–925. doi: 10.1093/aje/kwi123. discussion 926–918. [DOI] [PubMed] [Google Scholar]
- Lundstrom S, Haworth CM, Carlstrom E, Gillberg C, Mill J, Rastam M, et al. Trajectories leading to autism spectrum disorders are affected by paternal age: Findings from two nationally representative twin studies. Journal of Child Psychology and Psychiatry. 2010;51(7):850–856. doi: 10.1111/j.1469-7610.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- Olsen J, Zhu JL. Re: “Advanced parental age and the risk of autism spectrum disorder”. Am J Epidemiol. 2009;169(11):1406. doi: 10.1093/aje/kwp064. author reply 1406–1407. [DOI] [PubMed] [Google Scholar]
- Peipert JF, Bracken MB. Maternal age: An independent risk factor for cesarean delivery. Obstetrics and Gynecology. 1993;81(2):200–205. [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happé F, Plomin R, Ronald A. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5% and 1%) 2011. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- Saha S, Barnett AG, Foldi C, Burne TH, Eyles DW, Buka SL, et al. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med. 2009;6(3):e40. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasanfar R, Haddad SA, Tolouei A, Ghadami M, Yu D, Santangelo SL. Paternal age increases the risk for autism in an Iranian population sample. Molecular Autism. 2010;1(1):2. doi: 10.1186/2040-2392-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott FJ, Baron-Cohen S, Bolton P, Brayne C. The CAST (Childhood Asperger Syndrome Test): Preliminary development of a UK screen for mainstream primary-school-age children. Autism. 2002;6(1):9–31. doi: 10.1177/1362361302006001003. [DOI] [PubMed] [Google Scholar]
- Shelton JF, Tancredi DJ, Hertz-Picciotto I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res. 2010;3(1):30–39. doi: 10.1002/aur.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Mandy WP, Scourfield J. Measuring autistic traits: Heritability, reliability and validity of the Social and Communication Disorders Checklist. British Journal of Psychiatry. 2005;187:568–572. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- Weschler D, Golombok J, Rust S. Manual for the Weschler intelligence scale for children. 3rd ed Psychological Corporation; Sidcup, UK: 1992. [Google Scholar]
- Williams J, Allison C, Scott F, Stott C, Bolton P, Baron-Cohen S, et al. The Childhood Asperger Syndrome Test (CAST): Test-retest reliability. Autism. 2006;10(4):415–427. doi: 10.1177/1362361306066612. [DOI] [PubMed] [Google Scholar]
- Williams E, Thomas K, Sidebotham H, Emond A. Prevalence and characteristics of autistic spectrum disorders in the ALSPAC cohort. Developmental Medicine and Child Neurology. 2008;50(9):672–677. doi: 10.1111/j.1469-8749.2008.03042.x. [DOI] [PubMed] [Google Scholar]