Abstract
Objective
The purpose of this study was to determine the relationship of poor asthma control to bronchodilator response (BDR) phenotypes in children with normal spirometry.
Methods
Asthmatic children were assessed for clinical indices of poorly controlled asthma. Pre and post bronchodilator spirometry were performed and the percent BDR determined. Multivariate logistic regression assessed the relationship of the clinical indices to BDR at ≥8%, ≥10% and ≥12% BDR thresholds.
Results
There were 510 controller naïve, and 169 on controller medication. In the controller naïve population the mean age (± 1SD) was 9.5 (3.4), 57.1% were male, 85.7% Hispanic. Demographics were similar in both populations. In the adjusted profile, significant clinical relationships were found particularly to positive BDR phenotypes ≥10% and ≥12% versus negative responses including younger age, (odds ratios (OR) 2.0, 2.5; P <.05), atopy (OR 1.9, 2.6;P< .01), nocturnal symptoms in females (OR 3.4, 3.8;P< .01); beta2 agonist use (OR 1.7, 2.8;P< .01); and exercise limitation (OR 2.2, 2.5;P< .01) only in the controller naïve population.
Conclusions
The BDR phenotype ≥10% is significantly related to poor asthma control providing a potentially useful objective tool in controller naïve children even when prebronchodilator spirometry is normal.
Keywords: Pulmonary function, Pediatric, Bronchial asthma
I. Introduction
Because the clinical presentation of asthma represents a variety of different pathobiological processes, recent approaches have stressed the importance of characterizing asthma phenotypes (1–6). This could result in a better understanding of potential mechanisms leading to the clinical manifestations of asthma, and provide useful information helpful in making therapeutic decisions. Since asthma is thought to have a strong inflammatory basis, recent approaches have emphasized evaluation of biomarkers to define the inflammatory phenotype. Thus, Covar et al. have shown that induced sputum eosinophilia can provide useful information regarding treatment response, and measures of asthma control in children (2). However, induced sputum may not be a practical in-office marker of inflammation for the pediatric patient. More recently exhaled nitric oxide (eNO) has gained recognition as a potentially useful non-invasive tool in children for managing asthma therapy and is currently being utilized in the practice setting (7). However, eNO is not currently included as part of standard care as recommended by NAEPP guidelines (8), nor routinely useful when added to guideline criteria (9).
Because the clinical history may be unreliable, spirometry is currently considered essential for asthma diagnosis, severity stratification and monitoring asthma control in children ≥ 5 years (8). In spite of these recommendations, a national survey of primary care providers reported that only 21% use spirometry routinely (10). One reason may be that the specific guideline defined spirometric measures used to classify asthma severity and control, the forced expiratory volume in 1 second (FEV1) and the FEV1/forced vital capacity (FEV1/FVC) ratio, generally correlate poorly with symptom-based severity in children (11). This is in contrast to the relationship seen in adults (12).
The bronchodilator response (BDR), as a physiological response, has traditionally been used to define the presence of asthma (8). More recently the BDR has been shown to reflect biomarkers of eosinophilic inflammation, such as eNO (13, 14), bronchial (15) and sputum (2) eosinophilia, as well as being associated with atopy (5, 16) and bronchial hyper reactivity (17). The BDR has also been reported to be a good predictor of responsiveness to inhaled corticosteroids (ICS) (4), long term prognosis (4, 5), and may reflect airway remodeling (18). In addition, cluster analysis has suggested that the BDR is an important component in defining asthma phenotypes (6). In spite of its potential clinical importance there has been a paucity of published reports describing the clinical characteristics of asthmatic children with the BDR phenotype established at the baseline visit (3, 5, 19).
Our hypothesis is that the BDR, which may reflect both physiological and inflammatory biomarkers, measured at the baseline evaluation of asthmatic children, could characterize a phenotype that captures essential clinical and physiological parameters. Since most asthmatic children have normal pre bronchodilator spirometry regardless of severity classification (11), the purpose of our study was to determine the relationship of clinical characteristics of poor asthma control to BDR phenotypes as potential useful clinical tools in children with normal pre bronchodilator spirometry. Furthermore, we wanted to determine whether these clinical characteristics maintained significance across BDR threshold levels lower than the traditional ≥ 12%, which might also be useful for the clinician as suggested by several previous studies in children (5,19).
II. Methods
Patient population
Children participating in a school-based, low income, asthma mobile van program, the Breathmobile™(20), were referred for care during the five year period 2004–2008 from school nurses, community public health clinics, response to flyers, and an asthma questionnaire as previously described. (21) Criteria for the diagnosis of asthma by the asthma specialist included a previous history of recurrent coughing, wheezing, shortness of breath with rest or with exercise, symptomatic improvement following beta2 agonist use, and exclusion of other diagnoses (8). The diagnosis of asthma was made solely on clinical grounds without the result of spirometry. An exacerbation was defined as a severe attack with respiratory distress in the previous year which would require increased beta2 agonist use and systemic corticosteroids, if available. A steroid bust at baseline describes a 3–5 day course of an oral corticosteroid given because the patient was very poorly controlled at the initial or baseline visit. Patients not receiving controller medication in the 6–8 weeks prior to initial evaluation were considered controller naïve. Those who had been receiving controller medication regularly, including the previous 6–8 weeks before the visit, were considered to be the “on controller” medication group. Patients were excluded from BDR evaluation if they had received beta2 agonist use within four to six hours of spirometry, but not with a history of recurrent upper respiratory infection. Only patients diagnosed clinically as having asthma with normal pre bronchodilator spirometry were included in this study.
IRB
IRB approval by the Children’s Hospital of Orange County IRB committee was waived because the retrospective data utilized for this study was de-identified prior to analysis. An informed consent for the BDR was not deemed necessary because the BDR was considered standard for assessing pulmonary function. However, all patients signed an institutional consent after appropriate explanation that permits standard evaluation.
Spirometry
Pulmonary function testing was attempted in children five years of age or older in a standing position, as previously reported (19). Spirometric results were included in the analysis only if the child completed at least three baseline FVC maneuvers that met American Thoracic Society (ATS) criteria in a maximum of six attempts and was able to successfully complete post-bronchodilator spirometry (22). The best spirometric measures of at least three attempts were recorded for analysis, including FVC, FEV1, FEV1/FVC ratio, and the forced expiratory flow (FEF) 25–75 predicted. Post-bronchodilator spirometry was evaluated 10 minutes after administering two puffs (180 mcg) from an albuterol meter dose inhaler (MDI) with a spacer. Complete and acceptable spirometric measures were compared to the Knudson/Intermountain Thoracic Society (IMTS) normal predictive value and adjusted for ethnic values based on a parent report of ethnicity or race. (23) Further details of methodology have previously been published (19). The BDR was calculated as follows: FEV1 L post bronchodilator minus FEV1 L pre-bronchodilator/FEV1 L pre-bronchodilator X 100%. Normal pre bronchodilator spirometry was defined as the 95th percent confidence interval for age and gender which establishes the lower limits of normal. For children 5 to 18 years, this was an FEV1 ≥ 80% and FEV1/FVC ratio ≥ 80% (24).
Skin tests
Screening skin prick tests to common inhalant indoor and outdoor allergens were performed by a nurse and assessed by the Breathmobile™ physician. A positive skin test was defined as a wheal ≥ 3 mm greater than the negative saline control. The patient was considered atopic if positive to at least one aeroallergen.
Data entry
We entered all patient data in a standardized format using the AsmaTrax, a computer based data entry program developed and trademarked by Dr. Loran Clement, formerly of Southern California Breathmobile™ program (20). The data included demographics, Impairment factors, including daytime symptom frequency (none per week to >1×/day), nocturnal symptoms (none per month to nightly), exercise limitation (none to usually), beta2 agonist use six to eight weeks prior to evaluation (none per week to >1×/day), and school absenteeism. Risk factors assessed included previous year exacerbations, emergency room department (ED) visits, hospitalizations, and the need for a steroid burst at the baseline visit.
Statistical Analysis
Logistic regression analysis assessed the relationship of the clinical indices consistent with guideline-defined Impairment domain cut points for poor asthma control to BDR including daytime symptoms ( > 2 days per week), nocturnal awakening ( > 2 nights per month), exercise limitations (minimal/sometimes/usually), beta2 agonist use ( > 2 days per week), and school absenteeism ( ≥ 5 days). Risk domain factors included prior year exacerbations (≥ 2 days), ED visits/hospitalization (any), and steroid burst at baseline visit. The relationship of these clinical parameters to BDR was assessed at the ≥8%, ≥10% and ≥12% threshold levels.
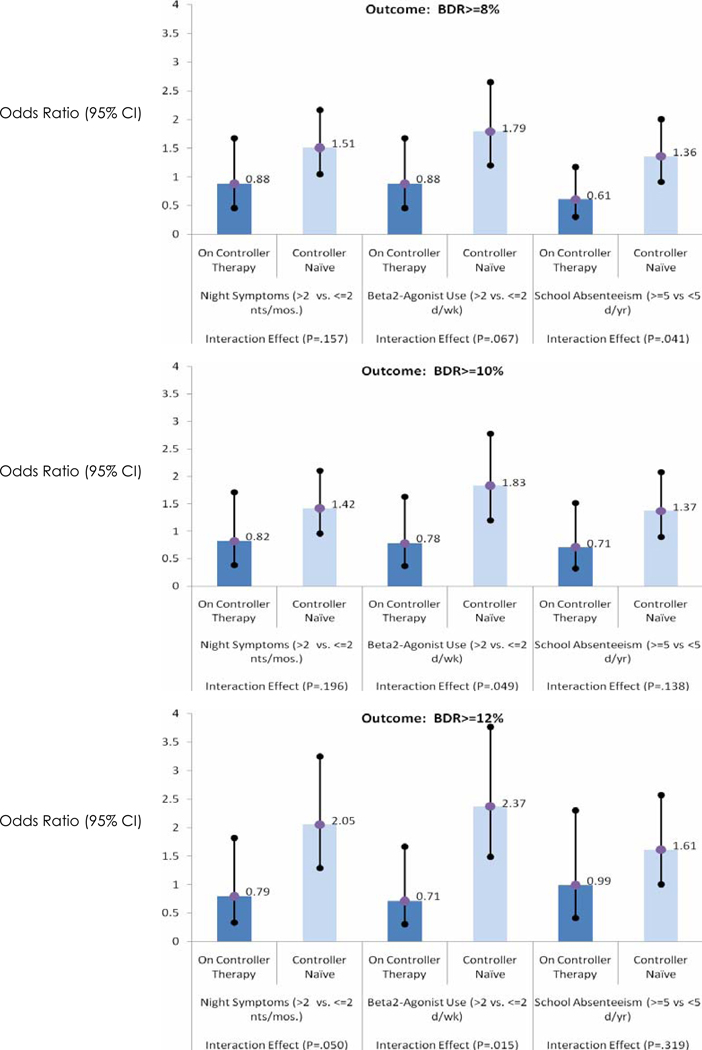
Univariate (unadjusted profile) and multivariate logistic regression (adjusted profile) analyses were used to establish the unadjusted and adjusted odds ratio (OR) to predict the presence of the BDR related to these clinical characteristics. Controller therapy status influences the relationship between certain Impairment and Risk domains on the likelihood of positive BDR, as displayed in Figure 1. A stratified approach was applied and the multivariate analysis powered within our predominately controller naïve population. Age was forced into each multivariate model with stepwise selection criteria applied and all two-way interaction terms investigated to determine the combination of factors that correspond to a positive BDR phenotype. All analyses were conducted using SPSS V12.0.
Figure 1.
Comparison of poor asthma control factors in controller naïve with on controller subset. Controller therapy status significantly impacts relationship between night symptoms, beta2-agonist use, and school days missed on likelihood of positive BDR at one or more threshold levels, unadjusted for other factors (p<.05). Non-significant subset differences were found for the following factors: atopy, family history, day symptoms, exercise limitations, steroid bursts, exacerbations, and emergency department visits/hospitalizations. View non-significance with caution due to limited number of patients on controller therapy at baseline.
III. Results
Demographic and clinical data, the latter consistent with guideline Impairment and Risk characteristics of poor asthma control, are shown in children with normal pre bronchodilator spirometry (Table I). Of the total population of 892 children, 679 (76%) had normal pre bronchodilator spirometry and are represented in Table I. The 679 children are divided into 510 who were controller naïve, and 169 who were receiving controller medication. In the controller naïve group the mean ± standard deviation (SD) age at baseline was 9.5 years (3.4), age of onset 4.4 years (4.1), 57.1% were males and 85.7% were Hispanics. The Basic Mass Index (BMI) measure revealed that approximately 30% of children were obese. Parental smoking was found in 18% of the children, atopy at 67.7%, average BDR was 5.9 (SD 7.8), FEV1 predicted 100% (12.4), FEV1/FVC 91% (6.1), and FEF25–75 101% of predicted (24.2). Impairment criteria consistent with poor control included day symptoms 49.8%, night symptoms 48.4%, exercise limitation (sometimes 31.1% and usually 35.1%), beta2 agonist use 29.0%, and school absenteeism, ED/Hospitalizations and higher average FEV1 and FEF25–75 predicted. In addition, of those children hospitalized approximately 10% of these admissions required the pediatric intensive care unit.
Table 1.
Demographic and BA characteristics of patients with normal pulmonary function (N=679).
| Column (valid %) | ||
|---|---|---|
| Controller Naïve | On Controller Therapy | |
| No. Patients | N=510 (75%) | N=169 (25%) |
| Demographic Characteristics | ||
| Age at baseline (y) mean (SD) | 9.5 yrs (3.4) | 9.2 yrs (3.4) |
| 5–6 years | 125 (24.5%) | 48 (28.4%) |
| 7–9 years | 144 (28.2%) | 51 (30.2%) |
| 10–18 years | 241 (47.3%) | 70 (41.4%) |
| Age at asthma onset (y) | 4.4 yrs (4.1) | 4.0 yrs (3.1) |
| Gender: | ||
| Male | 291 (57.1%) | 106 (62.7%) |
| Female | 219 (42.9%) | 63 (37.3%) |
| Ethnicity: | ||
| Hispanic | 437 (85.7%) | 133 (78.7%) |
| Caucasian | 29 (5.7%) | 19 (11.2%) |
| Other | 44 (8.6%) | 17 (10.1%) |
| *BMI percentile: | ||
| Normal (<85%) | 223 (44.7%) | 86 (53.1%) |
| Overweight (85–94%) | 115 (23.0%) | 29 (17.9%) |
| Obese (>=95%) | 161 (32.3%) | 47 (29.0%) |
| BA Characteristics | ||
| Family History | 204 (40.0%) | 69 (40.8%) |
| Parental Smoking | 83 (17.9%) | 27 (17.6%) |
| Atopic(any + ST) a‡ | 315 (66.7%) | 118 (79.7%) |
| BDR mean (SD) | 5.9 (7.8) | 4.7 (7.1) |
| FEV1% -predicted ‡ | 100% (12.4) | 104% (13.4) |
| FEV1/FVC | 91% (6.1) | 92% (6.3) |
| FEF25–75%-predicted† | 101% (24.2) | 106% (25.7) |
| Impairment Factors: | ||
| Day Sx (>2 d/wk) | 252 (49.8%) | 96 (57.1%) |
| Night Sx (>2 nt/mos.) | 244 (48.4%) | 86 (51.2%) |
| Exercise limitations: | ||
| Sometimes | 156 (31.1%) | 53 (32.1%) |
| Usually | 176 (35.1%) | 55 (33.3%) |
| Beta2-agonist use (>2 d/wk) ‡ | 146 (29.0%) | 83 (49.4%) |
| School absenteeism (>=5 d) ‡ | 150 (29.4%) | 72 (42.6%) |
| Risk Factors (year prior): | ||
| Steroid Burst at Baseline Visit | 38 (7.5%) | 6 (3.6%) |
| Exacerbations (>=2) | 110 (21.6%) | 46 (27.2%) |
| ED visit/hospitalization (>=1) ‡ | 189 (37.1%) | 100 (59.2%) |
P≤.05,
P≤.01 (Significance of distributional differences between controller therapy groups tested by chi-square test (categorical variables) and independent t-test (continuous variables).
Skin test performed in 88% of patients on therapy and 91% of controller naïve patients.
Skin tests: Cat, dog, feather, cockroach, mites, molds, weeds, trees, grass.
BMI: Body Mass Index
The unadjusted profile of controller naïve children with a positive BDR defined by 3 thresholds; ≥8%, ≥10% and ≥12% is shown in Table II. Thirty six percent of this subset demonstrated the ≥8% phenotype, while 27% and 19% demonstrated the ≥10% and ≥12% BDR phenotypes, respectively. Young children (5–6 years) at baseline and those younger at onset of asthma had greater likelihood of positive BDR at all thresholds with OR of 2.0 (1.1–3.3 95% CI) and 0.9 (0.8, 1.0 95% CI), respectively, (P< .01). There were no significant differentials of positive BDR observed by gender, BMI, family history or parental smoking. Significant influence was observed for atopy at the ≥10% and ≥12% threshold with OR of 1.7 (1.1, 2.7) (P< .05) and 2.0 (1.1, 3.4) (P< .01).
Table II.
Controller Naïve and Normal Pulmonary Function. Unadjusted Profile of patients with positive BDR defined by three threshold values.
Unadjusted Odds Ratio (OR) ~ likelihood of +BDR in patients defined by each characteristic compared to the reference category.
| Row % (valid) | BDR Threshold Value Classifying + Response |
||
|---|---|---|---|
| BDR>=8% | BDR>=10% | BDR>=12% | |
| N=185 (36%) | N=137 (27%) | N=95 (19%) | |
| Demographic Characteristics: | |||
| Age: | |||
| 5–6 years (reference) | reference | reference | reference |
| 7–9 years | 0.8 (0.5,1.3) | 1.0 (0.6,1.6) | 1.2 (0.7,2.1) |
| 10–18 years | 0.5 (0.3,0.8) ‡ | 0.5 (0.3,0.9) ‡ | 0.5 (0.3,0.8) ‡ |
| Age at onset ~ mean a | 0.9 (0.9,1.0) ‡ | 0.9 (0.9,1.0) ‡ | 0.9 (0.8,1.0) ‡ |
| Female vs. Male | 0.9 (0.6,1.2) | 1.0 (0.7,1.4) | 0.9 (0.6,1.4) |
| Hispanic vs. Other | 1.6 (0.9,2.8) | 1.3 (0.7,2.2) | 1.5 (0.7,3.1) |
| BMI percentile: | |||
| Normal (<85%) | reference | reference | reference |
| Overweight (85–94%) | 1.0 (0.6,1.6) | 0.9 (0.6,1.6) | 1.1 (0.6,1.8) |
| Obese (>=95%) | 1.0 (0.6,1.5) | 0.9 (0.5,1.4) | 0.9 (0.5,1.5) |
| BA Characteristics | |||
| Family History (Yes vs. No) | 0.8 (0.5,1.1) | 1.1 (0.7,1.6) | 1.1 (0.7,1.8) |
| Atopic vs. Non-atopic | 1.2 (0.8,1.8) | 1.7 (1.1,2.7) † | 2.0 (1.1,3.4) ‡ |
| Impairment Factors: | |||
| Day Sx (>2 vs. <=2 d/wk) | 1.1 (0.8,1.6) | 1.1 (0.7,1.7) | 1.5 (1.0,2.3) |
| Night Sx (>2 vs. <=2 nt/mos) | 1.5 (1.1,2.2) † | 1.4 (1.0,2.1) | 2.1 (1.3,3.3) ‡ |
| Exercise limitations : | |||
| Sometimes vs. None-Rarely | 1.5 (1.0,2.3) | 2.0 (1.2,3.3) ‡ | 2.2 (1.2,3.8) ‡ |
| Usually vs. None-Rarely | 1.0 (0.7,1.6) | 1.1 (0.7,1.9) | 1.2 (0.7,2.2) |
| Beta2-agonist use (>2 vs. <=2 d/wk) | 1.8 (1.2,2.6) ‡ | 1.8 (1.2,2.8) ‡ | 2.4 (1.5,3.8) ‡ |
| School absenteeism (>=5vs.<5 d/yr) | 1.4 (0.9,2.0) | 1.4 (0.9,2.1) | 1.6 (1.0,2.6) † |
| Risk Factors (year prior): | |||
| Steroid Burst at Baseline (Yes vs No) | 1.5 (0.8,2.9) | 2.1 (1.1,4.1) † | 2.8 (1.4,5.7) ‡ |
| Exacerbations (>=2 vs. <2) | 1.2 (0.8,1.8) | 1.4 (0.9,2.2) | 1.3 (0.8,2.2) |
| ED visit/hospitalization (>=1 vs. 0) | 1.4 (1.0,2.0) | 1.4 (0.9,2.0) | 1.4 (0.9,2.2) |
P≤.05,
P≤.01, logistic regression analysis.
For Impairment factors, nocturnal symptoms corresponded to increased likelihood of positive BDR at the ≥8% and ≥12% with OR of 1.5 (1.1, 2.2) (P < .05) and 2.1 (1.3, 3.3) (P< .01) respectively, and patients who sometimes vs. minimally experienced exercise limitation were twice as likely to have a positive BDR at the ≥10% and ≥12% thresholds (P< .01). Beta2 agonist use was statistically significant at all thresholds with OR’s ranging from 1.8 (1.2, 2.6) to 2.4 (1.5, 2.8) (P <.01), while school absenteeism was significant only at the ≥12% threshold with an OR of 1.6 (1.0, 2.6) (P < .05). For Risk factors, steroid bursts at baseline was a significant factor at the upper BDR thresholds OR 2.1 (1.1, 4.1) (P < .05) and 2.8 (1.4, 5.7) (P < .01). The unadjusted analysis in patients on controller medication showed no statistically significant relationship of clinical characteristics to the BDR at any of the thresholds (data not shown).
The relationship of clinical characteristics to the BDR phenotype in those on controller medication compared to the controller naïve population is shown in Figure 1. Graphical presentation restricted to characteristics that showed significant dependency on controller therapy status in terms of likelihood of positive BDR at any threshold level, is shown in Figure 1. Night symptoms, beta2 agonist use and school absenteeism all showed greater OR responses in the controller naïve group compared to those on controller therapy. This reached statistical significance for school absenteeism at the ≥8% BDR threshold, night symptoms at the ≥12% BDR threshold (P < .05), and beta2 agonist use at the BDR ≥10% (P <.05) and the ≥12% thresholds (P <.02). However, non significance of interaction term for other factors should be viewed with caution due to the limited number of patients in the controller treated subset as mentioned before.
The adjusted profile of patients with a BDR phenotype defined by the three threshold values at baseline is shown in Table III. Young age (5–6 vs. ≥10 years) remained a factor at all thresholds with an OR ranging from 2.5 (1.3, 5.0) (P <.01) to 2.0 (1.1, 3.3) (P <.05). Atopy also maintained significance after adjustment at the BDR ≥10% and ≥12% thresholds with OR’s of 1.9 (1.2, 3.1) (P <.01) and 2.6 (1.4, 4.9), respectively (P <.01). Night symptoms were significant at all thresholds in female patients only with OR ranging from 3.4 (1.2, 5.0) (P <.01) to 3.8 (1.8, 7.9) (P <.01). Beta2 agonist use continued to show significance at all thresholds with OR’s ranging from 1.7 (1.0, 2.9) (P <.01) to 2.8 (1.4, 5.7) (P <.01) with the latter dependent on less than 2 exacerbations in the previous year. Exercise limitation phenotype found previously (Table II) was maintained at the BDR ≥10% and ≥12% with OR’s ranging from 2.2 (1.3, 3.9) (P<.01) to 2.5 (1.3, 4.8) (P<.01) in the adjusted model. Parental smoking showed a small indirect influence at the lower thresholds, but not at the BDR ≥12% threshold. Adjusted analysis was limited in patients on controller medication because of inadequate number of patients in the subset.
Table III.
Controller Naïve and Normal Pulmonary Function: Adjusted Profile of patients with positive BDR defined by three threshold values at baseline. Interaction effects ~ adjusted odds ratios presented for BA characteristics at factor level(s) of interaction effect where significance detected.
Adjusted Odds Ratio (OR) a ~ likelihood of +BDR in patients defined by each characteristic compared to the reference category, adjusted for significant factors and with age forced into each model.
| Row % (valid) | BDR Threshold Value Classifying+ Response |
||
|---|---|---|---|
| Select significant factors presented a | BDR>=8% N=185 (36%) |
BDR>=10% N=137 (27%) |
BDR>=12% N=95 (19%) |
| Patient age at baseline: | |||
| Age 5–6 years (reference) | reference | reference | reference |
| Age 7–9 years | 0.7 (0.4,1.3) | 0.8 (0.5,1.5) | 1.2 (0.6,2.3) |
| Age 10–18 years | 0.5 (0.3,0.9) † | 0.5 (0.3,0.8) ‡ | 0.4 (0.2,0.8) ‡ |
| BA Characteristics: | |||
| Atopic vs. non-atopic |
Family History & Age of onset dependent |
1.9 (1.2,3.1) ‡ | 2.6 (1.4,4.9) ‡ |
| Night Symptoms: | Female patients: | Female patients: | Female patients: |
| (>2 vs. <=2 nt/month) | 3.8 (1.8,7.9) ‡ | 2.4 (1.2,5.0) † | 3.0 (1.2,7.7) † |
| Beta2-agonist use: | <2 exacerbations: | ||
| (>2 vs. <=2 d/wk) | 2.4 (1.3,4.4) ‡ | 1.7 (1.0,2.9) † | 2.8 (1.4,5.7) ‡ |
| Exercise limitations: | non-significant | ||
| Sometimes vs. none-rarely | 2.2 (1.3,3.9) ‡ | 2.5 (1.3,4.8) ‡ | |
P≤.05,
P≤.01, multivariate logistic regression analysis applying stepwise selection criteria in determining the combination of factors presented in Table 2 that correspond to increased likelihood of positive BDR.
Final Models (only clinically relevant significant factors in final models presented above).
IV. Discussion
Our data suggest that the BDR phenotype is associated with several important clinical characteristics of poor asthma control and atopy in those with normal pre bronchodilator spirometry, but only in the controller naïve subset. Nonetheless, this is a very useful observation because spirometry is the only objective in-office clinical tool the physician has, since the history is often unreliable and the physical examination normal when the child is asymptomatic. Unfortunately, pre bronchodilator sprirometry is usually in the normal range regardless of symptom based severity classification or asthma control (11). In our study population with normal spirometry up to 50% showed evidence of poor control, regardless of controller therapy status. In this situation the clinician could miss potentially critical information regarding bronchial lability (25) and associated poor asthma control if the BDR was not preformed. We found that 27% of children with normal prebronchodilator spirometry had a ≥10% BDR associated with clinical characteristics of poor control (Tables II and III).The traditional BDR threshold for a positive response is ≥12% and 200milliliters, but this was established primarily in adults (22). Sharma et al. reported that in children with mild to moderate asthma that a persistent positive BDR ≥12% over a 4 month or 4 year period predicted poor clinical and spirometric outcomes, particularly in those who had not received inhaled corticosteroids over that time frame (5). Of interest is that the BDR ≥10% gave similar results and was more useful because it increased identification of potentially high risk children from 52 to 84. This is similar to our findings where the clinical characteristics of both the BDR ≥10% and ≥12% were similar (Tables II and III), but the BDR ≥10% increased the potential number of children at risk from 95 to 137 (44%). The BDR ≥8% threshold also showed evidence of poor asthma control, but not as consistently as the higher thresholds (Tables II and III). The BDR ≥8% was included in our analysis because it was greater than the variability (95%CI) of the pre bronchodilator FEV1 evaluated in our population (data not shown). Furthermore, we have recently shown that the BDR ≥8% predicts bronchial airway inflammation as shown by the exhaled nitric oxide (eNO) biomarker with a positive predictive value of ≥80 % (14). It is obvious from our data that the optimal BDR threshold to detect poor asthma control has not been established, and nor was it the purpose of our study. Rather, as reported by Sharma et al. (5) we wanted to evaluate whether thresholds lower than BDR ≥12 % could demonstrate characteristics of poor control which would identify a larger cohort at risk. The BDR ≥10% appears to be a good compromise in terms of relationship to asthma control and identification of children at risk.
Although this study was not powered to compare the controller naïve and on controller populations, since only 20% of our inner city patient base enters the program on controller medication, we found a striking difference in the relationship of the BDR to clinical characteristics of poor control in these two subsets. Unlike the controller naïve group those on controller medication showed no significant relationship at any BDR threshold (data not shown). Furthermore, this population showed significantly lower OR to several important indications of poor control when directly compared to the controller naïve cohort (Fig 1.). This is in spite of the fact that those on controller medication were a more poorly controlled population on entry as shown by more beta2 agonist use, school absenteeism and ED visits/hospitalization (Table I.). There are several potential explanations for this rather paradoxical observation. The most obvious is inadequate numbers to adequately power the unadjusted and adjusted analysis. This reflects the standard of care of under treatment in the community of inner city children we serve. However, another plausible explanation is the sensitivity of the BDR to ICS. A number of studies have shown that the BDR is a good predictor of ICS responsiveness (4) and decreases in conjunction with bronchial eosinophilia in a time dependent fashion on ICS (15). We have previously shown a significant relationship between the BDR, eNO and asthma control (14). However this was only observed in the controller naïve population, since both the BDR and bronchial eNO were significantly reduced in those on ICS and were no longer related to asthma control. Alveolar eNO, which was not influenced by ICS and not related to BDR, had a consistent relationship to asthma control, regardless of controller therapy status (14). This could explain the disconnect between the BDR and control in the on controller subset. In our study the total number on controller who demonstrated the BDR phenotype was further lowered by including only those with normal pre bronchodilator spirometry. Thus, this population needs further studies with a larger cohort to determine to what extent, if any, the BDR would be a useful clinical marker over time once controller therapy is initiated.
In addition to the relationship of BDR to poor asthma control, atopy was a strong predictor of the BDR ≥10 % and ≥12 % in the controller naïve subset. This is consistent with previous observations relating atopy to the BDR (5, 16), asthma severity (26), bronchial hyper-reactivity (13, 17), induced sputum (2) and bronchial eosinophilia (15). This is clinically relevant since it directs the physician to consider environmental allergens in addition to pharmacotherapy in a more comprehensive approach to treatment.
Age was also a consistent predictor of the BDR phenotype with children 5–6 years of age being at least twice as likely to demonstrate the BDR phenotype at all thresholds. This finding is consistent with the report by Kumar et al. (3) in Chinese children who showed a decrease in BDR from ages 8–15 years, and Tantisira et al. who showed that bronchial hyper-reactivity decreases with age which was correlated with the BDR (17). In our study population the profile of asthma characteristics that define control corresponding to the likelihood of a positive BDR phenotype did not appear to be age dependent at any threshold (P > .05).
V. Limitations
Several potential limitations need to be addressed. This study was a retrospective, observational study with a cross sectional analysis which captured only a baseline “snapshot” of an ongoing process. While it will be critical to extend our findings over time, Sharma’s longitudinal study provides important insights relevant to our study (5). First, those with the persistent BDR phenotype (responders) were a small percentage of the total population at risk, approximately 5% of over 1,000 patients. This is in contrast to 27% with the BDR ≥10% in our population (Table II). It is important to note that in Sharma’s responder group the average BDR at baseline was 26% compared to 9% in the non responders (P< .001), suggesting that one might predict long term consequences by the baseline BDR as also shown by Tantisera et al. (4). Providing controller therapy to only those with the consistent BDR phenotype pattern over months to years could result in missing the opportunity to treat a much larger cohort at risk identified by the BDR phenotype at baseline.
Since our population was predominantly Hispanic, inner city, with poor access to medical care, one cannot necessarily generalize our findings to other populations since different genetic groups may respond differently to beta2 agonists (27). In addition, this was an observational study with patients enrolled on the basis of clinical need rather than a randomized research protocol. Therefore, there were no pre-selected entry criteria other than the ability to perform the BDR and the necessity to be diagnosed as asthmatic by the asthma specialist. The use of long acting beta2 agonist used in combination therapy within 24 hours of spirometry was not an exclusion criterion, but applied to only a very small percentage of our patient population. Finally, the assessment of the BDR 10 minutes after albuterol administration, because of patient flow concerns, does not represent the peak BDR (28), so that we probably underestimated the BDR in our population.
VI. Conclusions
The BDR phenotypes, particularly ≥10% and ≥12%, in children with normal pre bronchodilator spirometry are characterized by poorly controlled asthma with increased beta2 agonist use, nocturnal symptoms, exercise limitation and atopy in our predominately controller naïve group. The BDR ≥10% phenotype appears to have a similar relationship to poor control factors as the BDR ≥12%, but identifies 44% more patients at potential risk and need of controller therapy, as well as consideration of environmental allergens. If confirmed in other ethnic populations, the BDR ≥10% phenotype could provide the clinician with a very useful in-office, objective tool for assessing controller naïve asthmatic children particularly when pre bronchodilator spirometry is in the normal range and the history is unclear. Longitudinal data will be critical particularly in those children on controller therapy to establish the utility of the BDR for monitoring asthma control and making therapeutic decisions in all asthmatic children.
Acknowledgments
Grant Support:
California Wellness Foundation
Tobacco Settlement Revenue
Asthma Chronic Lung Disease Grant
Air Quality Management District Southern California
Abbreviations
- BDR
Bronchodilator Response
- NAEPP
National Asthma Education and Prevention Program
- OR
Odds ratios
- ICS
Inhaled corticosteroid
- eNO
exhaled nitric oxide
Footnotes
Conflict of interest: none real or perceived
REFRENCES
- 1.Wenzel SE. Asthma: Defining the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 2.Covar RA, Spahn JD, Martin R, Silkoff PE, Sundstrom BJ, Murphy J, et al. Safety and application of induced sputum analysis in childhood asthma. J Allergy Clin Immunol. 2004;114:575–582. doi: 10.1016/j.jaci.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Wang B, Wang X, Chen C, Yang J, Fu L, et al. Bronchodilator response in Chinese children from asthma index families and the general population. J Allergy Clin Immunol. 2006;117:1257–1263. doi: 10.1016/j.jaci.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 4.Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta MV, Zeiger RS, Strunk RL, et al. Bronchodilation and bronchocontriction: Predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006;117:1264–1271. doi: 10.1016/j.jaci.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, et al. Clinical predictors and outcomes of consistent bronchodilator response in Childhood Asthma Management Program. J Allergy Clin Immunol. 2008;122:921–928. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haldar P, Pavord TD, Shaw PE, Berg MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Amer J. Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitt M. Clinical applications of exhaled nitric oxide for the diagnosis and management of asthma: a consensus report. Clin Ther. 2005;27:1238–1250. doi: 10.1016/j.clinthera.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. Summary Report 2007. J Allergy Clin Immunol. 2007;120:S93–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner city adolescents and young adults; a randomized controlled trial. Lancet. 2008;372:1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finklestein JA, Lozano P, Shulruff R, Inui TS, Soumerai SB, Ng M, et al. Self reported physician practices for children with asthma: Are national guidelines followed? Pediatrics. 2000;106:886–896. [PubMed] [Google Scholar]
- 11.Bacharier LB, Strunk RC, Mauger D, White d, Lemanske RF, Sorkness CA. Classifying asthma severity in children. Mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;176:426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins HA, Cherniak RM, Szefler IJ, Covar R, Gelfand EW, Spahn JD. A comparison of the clinical characteristics of children and adults with severe asthma. Chest. 2003;124:1318–1324. doi: 10.1378/chest.124.4.1318. [DOI] [PubMed] [Google Scholar]
- 13.Covar RA, Szefler J, Martin RJ, Sundstrom DA, Silkoff DA, Murphy J, et al. Relations between exhaled nitric oxide and measures of airway disease among children with mild to moderate asthma. J Pediatr. 2003;142:469–475. doi: 10.1067/mpd.2003.187. [DOI] [PubMed] [Google Scholar]
- 14.Puckett JL, Taylor RWE, Szu-yun L, Guijon O, Aledia AS, Galant SP, et al. An elevated bronchodilator response predicts large airway inflammation in mild asthma. Pediatr. Pulmonol. 2010;45:174–181. doi: 10.1002/ppul.21172. [DOI] [PubMed] [Google Scholar]
- 15.Faul JL, Demers EA, Burke CM, Poulter LW. Alterations in airway inflammation and lung function during corticosteroid therapy for atopic asthma. Chest. 2002;121:1414–1420. doi: 10.1378/chest.121.5.1414. [DOI] [PubMed] [Google Scholar]
- 16.Marotta A, Klinnert MD, Price MR, Larson GL, Liu AH. Impulse oscillometry provides an effective test of lung dysfunction in 4 year old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:17–22. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 17.Tantisera KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbridge AC. Airway responsiveness in mild to moderate childhood asthma. Am J Respir Crit Care Med. 2008;178:325–331. doi: 10.1164/rccm.200708-1174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goleva E, Hauk PJ, Boguniewicz, Martin RJ, Leung DYM. Airway remodeling and lack of bronchodilator response in steroid-resistant asthma. J Allergy Clin Immunol. 2007;120:1065–1072. doi: 10.1016/j.jaci.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galant SP, Morphew T, Amaro S, Liao O. Characteristics of the bronchodilator response in controller naïve asthmatic children. J Pediatr. 2007;151:457–462. doi: 10.1016/j.jpeds.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Jones CA, Clement LT, Hanley-Lopez J, Morphew T, et al. The Breathmobile™ Program: Structural implementation and evaluation of a large scale, urban Pediatric Asthma Disease Management Program. Disease Management. 2005;8:205–221. doi: 10.1089/dis.2005.8.205. [DOI] [PubMed] [Google Scholar]
- 21.Galant SP, Crawford LJR, Morphew T, Jones CA, Bassin S. Predictive value of a cross-cultural asthma case-detection tool in an elementary school population. Pediatrics. 2004;114:e307–e316. doi: 10.1542/peds.2003-0575-F. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Lung function testing: selection of reference values and interpretative staratgies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 23.Knudson RJ, Lebowitz MD, Holbert CJ, Burrows B. Changes in the normal maximal expiratory flow volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 24.Stanojevic S, Wade A, Stocks J, Hankinson J, Coates AL, Pan H, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RS. Assessment of respiratory function in the asthmatic child. BR Med J. 1966;2:972–975. doi: 10.1136/bmj.2.5520.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman B, Feanny S, Reisman J, Hak H, Rashed N, McLaughlin FJ, et al. The Dose relationship of allergy to severity of childhood asthma. J Allergy Clin Immunol. 1988;81:63–70. doi: 10.1016/0091-6749(88)90221-7. [DOI] [PubMed] [Google Scholar]
- 27.Tsai HJ, Shaikan, Kito JY, Battle N, Naqui M, Navarro O, et al. Beta-2 adrenergic receptor polymorphisms: pharmacogenetic response to bronchodilator among African American asthmatics. Hum Genet. 2006;119:547–557. doi: 10.1007/s00439-006-0169-2. [DOI] [PubMed] [Google Scholar]
- 28.Stavreska V, Verheggen M, Oostryck J, Stick SM, Hall GL. Determining the time to maximal bronchodilator response in asthmatic children. J Asthma. 2009;46:25–29. doi: 10.1080/02770900802460555. [DOI] [PubMed] [Google Scholar]