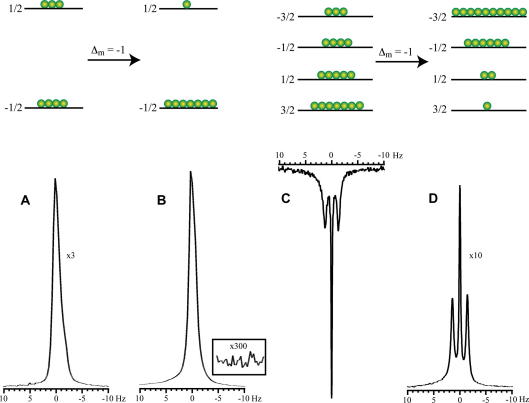
Fig. 2.
Gas phase NMR spectra collected at 11.7 T of 129Xe (A and B) and 131Xe (C and D). Also indicated are the associated energy levels at thermal, high temperature equilibrium (A and C) and after optical pumping using the transition Δm = −1 (B and D). (A) 129Xe NMR spectrum of xenon at 400 kPa (partial pressure) of pure xenon and 100 kPa (partial pressure) oxygen using 250 transients. (B) Hyperpolarized 129Xe NMR spectrum with 10 kPa (partial pressure) xenon from a 200 kPa, 5% xenon gas mixture after a single stopped-flow delivery. Note that the scale of (A) is threefold enlarged compared to (B) in this figure. (C) Thermal 131Xe NMR spectrum after 1260 transients with a partial pressure of 93 kPa of xenon from a 100 kPa, 93% xenon gas mixture and (D) hyperpolarized 131Xe NMR spectrum after a single stopped-flow delivery using 10 kPa xenon partial pressure of a 200 kPa, 5% xenon gas mixture. The scale of (D) is 10-fold enlarged compared to (C). All hyperpolarized spectra are collected with one transient. When correcting for xenon partial pressures and number of transients, enhancements of 33,000 (corresponding to 37% spin polarization) and 1500 (i.e. 0.8% spin polarization) were achieved for 129Xe and 131Xe, respectively. Note, that up to 2.2% 131Xe spin polarization was obtained in later experiments shown in Fig. 4. The differences in the relative phase are due to the positive gyromagnetic ratio of 131Xe, as discussed in Section 3.3. All spectra were recorded using xenon with natural abundance isotope distribution.