Abstract
The Cape Floral Region represents one of the world’s biodiversity hot spots, with a high level of plant, animal and insect endemism. The fungi occurring in this region, however, remain poorly studied. It is widely postulated that each plant species should harbour at least five to six unique fungal species, a number that we regard to be a huge underestimate. To test this hypothesis, we decided to study a single senescent flower of Phaenocoma prolifera (‘everlasting’; Asteraceae) collected in South Africa, and posed the question as to how many different species of fungi could be isolated and cultivated from 10 leaf bracts. Using a damp chamber technique, numerous microfungi could be induced to sporulate, enabling most of them to be successfully isolated on artificial agar media. Isolates were subsequently subjected to DNA sequencing of the ITS and LSU nrDNA regions. During the course of this study 17 species could be cultivated and identified, of which 11 appeared to be new to science. These include Catenulostroma hermanusense, Cladosporium phaenocomae, Devriesia tardicrescens, Exophiala capensis, Penidiella aggregata, P. ellipsoidea, Teratosphaeria karinae, Toxicocladosporium pseudoveloxum spp. nov., and Xenophacidiella pseudocatenata gen. & sp. nov. Further studies are now required to determine if these fungi also occur as endophytes in healthy flowers. If this trend holds true for other plant hosts from southern Africa, it would suggest that there are many more fungi present in the Cape Floral Region than estimated in previous studies.
Keywords: Batcheloromyces, Catenulostroma, Cladosporium, Devriesia, Exophiala, ITS, LSU, Penicillium, Penidiella, Phaenocoma prolifera, systematics, Teratosphaeria, Toxicocladosporium, Xenophacidiella
INTRODUCTION
Fynbos, which is essentially shrubland vegetation, is the most characteristic vegetation type of the Cape Floral Region, which is the world’s richest and most diverse floristic region (Goldblatt 1997). The Cape Floral Region has an extremely high level of species richness and endemism, which has been attributed to either a combination of diverse habitats and steep ecological gradients, or an intermediate level of system stress in the Cape Floral Region (Davis et al. 1994, Goldblatt 1997). Approximately 68 % of the species, 20 % of the genera and six families are endemic to the region (Bond & Goldblatt 1984, Goldblatt 1997). The largest concentration (70 %) of southern Africa’s Red Data Book plants, occurs in the Cape Town metropolitan area with 15.1 species per km2 (Hilton-Taylor 1996).
Taylor (1977) drew attention to the decline of the Cape Floral Region, by reporting a close to 60 % reduction in size of the area due to agricultural development, industry, urbanisation, intrusion of alien invasive plants, deforestation and fragmentation. Retaining the biodiversity in the fynbos is economically important, as a number of species are used in the wildflower industry (Crous et al. 2004a), and for the production of thatching materials (Wessels et al. 1997), and ecotourism (Davis et al. 1994, Cowling et al. 1997).
One unique example of a fynbos species is the monotypic genus Phaenocoma (Asteraceae). Phaenocoma is based on P. prolifera (commonly referred to as Cape strawflower, Cape everlasting, or ‘Rooi sewejaartjie’ in Afrikaans), which is restricted to the Western Cape Province of South Africa. The name Phaenocoma refers to the shiny leaf bracts (‘phaino’: to shine, and ‘coma’: hair) (Jackson 1990; www.sanbi.org).
These plants are common in the Cape Floristic Region, occurring on sandy soils on mountain slopes and in valleys, at altitudes ranging from sea level to 1 500 m, where they grow as shrubs that can become up to 1.2 m tall. Plants flower from September to January, forming terminal flower heads which contain up to 1 000 individual flowers with bright pink to red bracts. The latter eventually fade, becoming white with age (Jackson 1990, Koekemoer 2002).
During a recent collection trip to the Western Cape Province, several senescent, but still attached flowers of P. prolifera were collected on the mountain slopes of the Fernkloof Nature Reserve, Hermanus, which were dirty-white in colour, with blackened stems. The aim of this study was thus to determine which fungi were colonising these senescent flowers. A further aim was to determine if any of these fungi had previously been reported to colonise other hosts in the Cape Floral Region, as has been found for some species occurring on Protea (Crous et al. 2008a, Marincowitz et al. 2008a, b) and Encephalartos (Crous et al. 2008b). Finally, by choosing a single flower head from this location, and only looking at leaf bracts of this flower, we wanted to know if we would obtain the five to six unique fungal species postulated by Hawksworth (1991) to occur on each species of flowering plants.
MATERIALS AND METHODS
Isolates
Ten flower bracts from a single flower were selected for study. Flower bracts bearing ascomata were soaked in water for approximately 2 h, after which they were placed in the inner side of Petri dish lids, of plates containing 2 % malt extract agar (MEA; Crous et al. 2009c). Ascospore germination patterns were examined after 24 h, and single ascospore and conidial cultures established as described earlier (Crous et al. 1991, Crous 1998). Flower bracts were also incubated in moist chambers for up to 2 wk, and inspected daily for microfungi, and single conidial colonies of hyphomycetes and coelomycetes established on MEA (Crous 2002). Colonies were subcultured onto synthetic nutrient-poor agar (SNA), potato-dextrose agar (PDA), oatmeal agar (OA), MEA (Crous et al. 2009c), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation.
DNA isolation, amplification and analyses
Genomic DNA was isolated from fungal mycelium grown on MEA, using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Solana Beach, CA, USA) according to the manufacturer’s protocols. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part of the nuclear rDNA operon spanning the 3′ end of the 18S rRNA gene (SSU), the internal transcribed spacer 1, the 5.8S rRNA gene, the internal transcribed spacer 2 (ITS) and the first 900 bases at the 5′ end of the 28S rRNA gene (LSU). The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009b) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. The PCR conditions, sequence alignment (using the online interface of MAFFT (mafft.cbrc.jp/alignment/server/index.html; Katoh et al. 2002), followed by manual correction by eye) and subsequent phylogenetic analysis (using PAUP v4.0b10; Swofford 2003) followed the methods of Crous et al. (2006a, 2009a). Partial actin (ACT) and translation elongation factor 1-alpha sequences were determined for Cladosporium spp. as described in Schubert et al. (2007) and Bensch et al. (2010). Sequences were compared with the sequences available in NCBIs GenBank nucleotide (nr) database using a megablast search and results are discussed in the relevant species notes where applicable. Alignment gaps were treated as new character states. Sequences derived in this study were lodged at GenBank, the alignment in TreeBASE (www.treebase.org), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004b).
Morphology
Microscopic preparations were made in clear lactic acid, with 30 measurements determined per structure, and observations made with a Nikon SMZ1500 dissecting microscope, and with a Zeiss Axioscope 2 microscope using differential interference contrast (DIC) illumination. Colony characters and pigment production were noted after 2 wk of growth on MEA, PDA, SNA and OA; Crous et al. 2009c) incubated at 25 °C. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970). Growth characteristics were studied on MEA plates incubated for 1–2 wk in the dark at 25 °C.
RESULTS
Isolations
During the present study a total of 50 taxa were isolated, which eventually were identified as representing 17 different species. A minimum of two single conidial or ascospore isolates was preserved of each isolated taxon in the CPC working collection of P.W. Crous, of which one isolate was subjected to DNA analysis. Reference strains are maintained in the CBS-KNAW Fungal Biodiversity Centre (CBS) Utrecht, The Netherlands. Isolates used for morphological and sequence analyses are presented in Table 1.
Table 1.
Collection details and GenBank accession numbers of isolates for which novel sequences were generated in this study.
| Species | Strain no. 1 | Substrate | Country | Collector(s) | GenBank Accession number |
|||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | TEF | ACT 2 | |||||
| Batcheloromyces leucadendri | CPC 18277 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499832 | JF499852 | – | – |
| Catenulostroma hermanusense | CBS 128768 = CPC 18276 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499833 | JF499853 | – | – |
| Cladosporium cladosporioides | CPC 18230 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499834 | JF499854 | JF499872 | JF499878 |
| Cladosporium perangustum | CPC 18228 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499835 | JF499855 | JF499873 | JF499879 |
| CPC 18229 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499836 | JF499856 | JF499874 | JF499880 | |
| Cladosporium phaenocomae | CBS 128769 = CPC 18223 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499837 | JF499857 | JF499875 | JF499881 |
| CPC 18221 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499838 | JF499858 | JF499876 | JF499882 | |
| Cladosporium ramotenellum | CPC 18224 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499839 | JF499859 | JF499877 | JF499883 |
| Devriesia tardicrescens | CBS 128770 = CPC 18259 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499840 | JF499860 | – | – |
| Exophiala capensis | CBS 128771 = CPC 18473 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499841 | JF499861 | – | – |
| Penidiella aggregata | CBS 128772 = CPC 18278 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499842 | JF499862 | – | – |
| Penidiella ellipsoidea | CBS 128773 = CPC 18317 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499843 | JF499863 | – | – |
| Teratosphaeria cf. bellula | CPC 18280 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499844 | JF499864 | – | – |
| CPC 18281 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499845 | JF499865 | – | – | |
| Teratosphaeria karinae | CBS 128774 = CPC 18255 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499846 | JF499866 | – | – |
| Toxicocladosporium pseudoveloxum | CBS 128775 = CPC 18257 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499847 | JF499867 | – | – |
| CBS 128777 = CPC 18471 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499848 | JF499868 | – | – | |
| CPC 18274 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499849 | JF499869 | – | – | |
| Xenophacidiella pseudocatenata | CBS 128776 = CPC 18472 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499850 | JF499870 | – | – |
| CPC 18279 | leaf bracts of Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499851 | JF499871 | – | – | |
1 CBS: CBS Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of P.W. Crous, housed at CBS.
2 ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA; LSU: partial 28S nrDNA; TEF: partial translation elongation factor 1-alpha gene; ACT: partial actin gene.
Phylogeny
Approximately 1 700 bases, spanning the ITS and LSU regions, were obtained from the sequenced cultures and approximately 450 and 230 bases for TEF and ACT, respectively. Three phylogenetic analyses were performed: 1) an analysis of the LSU region to determine the generic relationship of the obtained isolates; 2) an analysis of the ITS sequences clustering in the Teratosphaeriaceae to confirm species-level relationships; and 3) a combined ITS, ACT and TEF analysis focussed on the Cladosporium isolates.
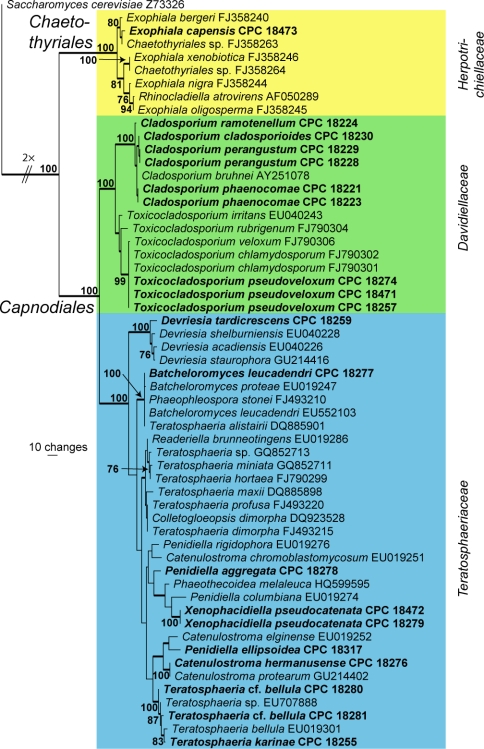
The manually adjusted LSU alignment contained 57 taxa (including the Saccharomyces cerevisiae outgroup sequence, GenBank Z73326) and, of the 840 characters (including alignment gaps) used in the phylogenetic analysis, 209 were parsimony-informative, 63 were variable and parsimony-uninformative and 568 were constant. The first of 153 equally most parsimonious trees retained from the heuristic search is shown in Fig. 1 (TL = 607, CI = 0.636, RI = 0.912, RC = 0.580). The phylogenetic tree of the LSU region (Fig. 1) shows that the obtained sequences cluster in Chaetothyriales and Capnodiales, with the latter mainly having associations with members of Davidiellaceae and Teratosphaeriaceae.
Fig. 1.
The first of 153 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment. The scale bar shows 10 changes and bootstrap support values > 75 % from 1 000 replicates are shown at the nodes. Thickened branches represent those present in the strict consensus tree. Novel sequences generated in this study are shown in bold and coloured boxes reflect different families. The tree was rooted to Saccharomyces cerevisiae (GenBank Z73326).
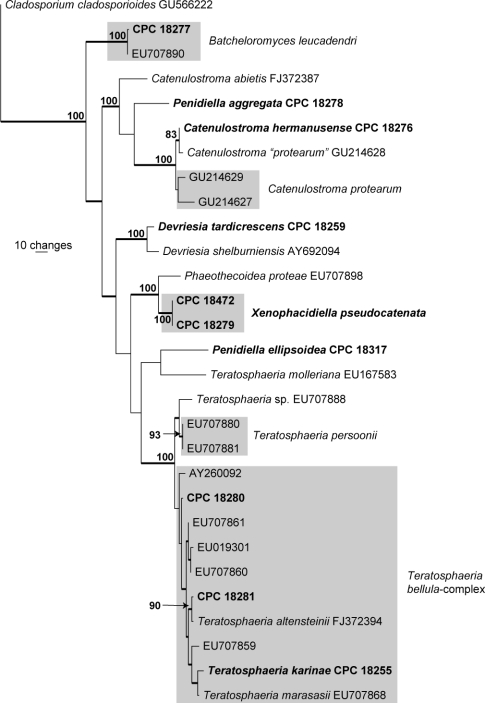
The manually adjusted ITS alignment contained 29 taxa (including the Cladosporium cladosporioides outgroup sequence, GenBank GU566222) and, of the 499 characters (including alignment gaps) used in the phylogenetic analysis, 167 were parsimony-informative, 56 were variable and parsimony-uninformative and 276 were constant. The first of four equally most parsimonious trees retained from the heuristic search is shown in Fig. 2 (TL = 596, CI = 0.596, RI = 0.757, RC = 0.451). Specific associations based on this tree are discussed, where applicable, under the species notes below.
Fig. 2.
The first of four equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the ITS sequence alignment. The scale bar shows 10 changes and bootstrap support values > 75 % from 1 000 replicates are shown at the nodes. Thickened branches represent those present in the strict consensus tree. Novel sequences generated in this study are shown in bold and grey boxes indicate those species with more than one strain present. The tree was rooted to Cladosporium cladosporioides (GenBank GU566222).
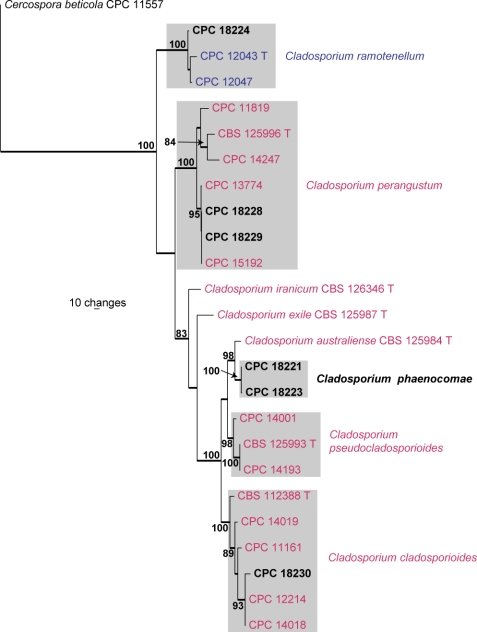
The manually adjusted combined ITS/ACT/TEF alignment contained 25 taxa (including the Cercospora beticola outgroup sequence, GenBank AY840527, AY840458, AY840494, respectively) and, of the 1 102 characters (including alignment gaps) used in the phylogenetic analysis, 230 were parsimony-informative, 212 were variable and parsimony-uninformative and 660 were constant. The first of six equally most parsimonious trees retained from the heuristic search is shown in Fig. 3 (TL = 596, CI = 0.596, RI = 0.757, RC = 0.451). Specific associations based on this tree are discussed, where applicable, under the species notes below.
Fig. 3.
The first of six equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the combined ITS/ACT/TEF sequence alignment. The scale bar shows 10 changes and bootstrap support values > 75 % from 1 000 replicates are shown at the nodes. Thickened branches represent those present in the strict consensus tree. Novel sequences generated in this study are shown in bold and grey boxes indicate those species with more than one strain present. Sequences of numbers in blue were generated by Schubert et al. (2007), and in red by Bensch et al. (2010). The tree was rooted to Cercospora beticola (GenBank AY840527, AY840458, AY840494, respectively).
Taxonomy
Seventeen fungal species were isolated from the 10 Phaenocoma leaf bracts studied. Known species included two species of Penicillium (not treated in the present manuscript), namely P. crocicola (= P. thomii) (DTO 132D6 = DTO 133G5) and a species recently described from fynbos soils, P. ramulosum (DTO 133G6 = DTO 133G7) (Visagie et al. 2009). Known species of Cladosporium that occurred on the leaf bracts include C. cladosporioides (CPC 18230), C. perangustum (CPC 18228, 18229), and C. ramotenellum (CPC 18224) (Fig. 3; discussed in Bensch et al. 2010). Teratosphaeria bellula (CPC 18280, 18281) and Batcheloromyces leucadendri (CPC 18277), were also isolated, as well as unknown, potentially undescribed species. These are treated and discussed below.
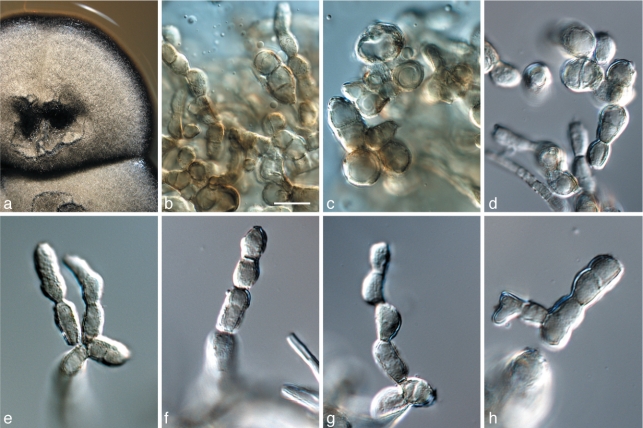
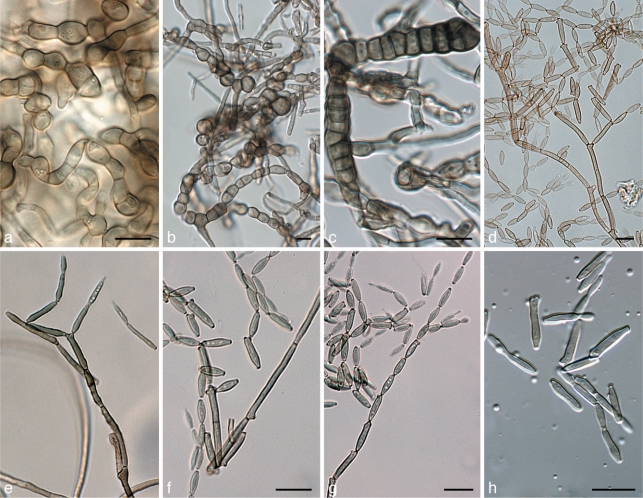
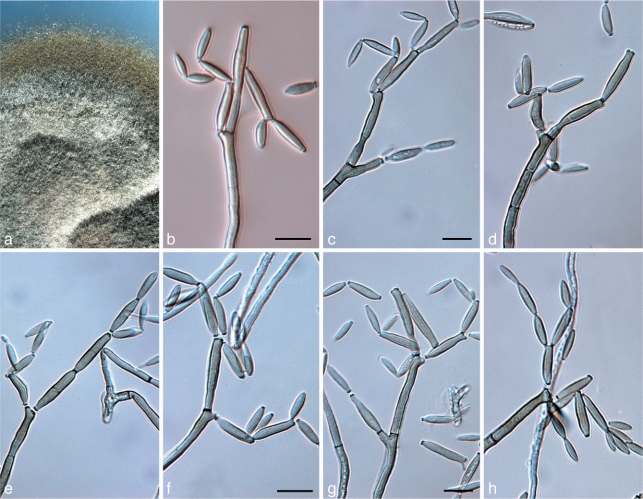
Catenulostroma hermanusense Crous, sp. nov. — MycoBank MB560017; Fig. 4
Fig. 4.
Catenulostroma hermanusense (CPC 18276). a. Colony on MEA; b–h. a series of conidiophores with chains of disarticulating conidia. — Scale bar = 10 μm.
Catenulostromatis protearum simile, sed conidiis minoribus, 10–25 × 5–10 μm.
Etymology. Named after the locality where it was collected, Hermanus, South Africa.
Colonies sporulating on MEA. Mycelium consisting of branched, septate, verruculose to warty, medium to dark brown, 2–4 μm wide hyphae. Conidiophores reduced to conidiogenous cells integrated on hyphal ends. Conidiogenous cells subcylindrical, unbranched, medium brown, 10–18 × 3–4 μm, thick-walled, with 1(–3) terminal loci; scars inconspicuous, 2–3 μm wide. Conidia in simple or branched chains, subcylindrical to ellipsoid, straight to flexuous, (10–)15–20(–25) × 5–8(–10) μm, 0–3 transversely septate, or with 1–2 oblique septa, medium to dark brown, thick-walled, verruculose to warty; hila unthickened, 2–3 μm wide.
Culture characteristics — Colonies spreading, erumpent, with folded surface and sparse aerial mycelium and even, smooth, crenate margins. On PDA surface olivaceous-grey, margin submerged, iron-grey; reaching 15 mm diam after 2 wk. On MEA similar in colour, also reaching 15 mm diam after 2 wk.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20528 holotype, cultures ex-type CPC 18276 = CBS 128768.
Notes — Phylogenetically (Fig. 2), C. hermanusense is closely related to C. protearum (Crous et al. 2009b), but is morphologically distinct in that it has smaller conidia (10–25 × 5–10 μm) than C. protearum (12–45 × 7–25 μm; Crous et al. 2007a). Our ITS sequence of C. hermanusense differs with three nucleotides from an isolate from Hakea (GenBank GU214628), originally assumed to belong to C. protearum. However, based on the current data it is possible that that isolate either belongs to C. hermanusense or represents a cryptic species closely related to it rather than to C. protearum. More isolates should be collected from both hosts and be subjected to multilocus sequence typing to test this hypothesis.
Cladosporium phaenocomae Crous, sp. nov. — MycoBank MB560018; Fig. 5
Fig. 5.
Cladosporium phaenocomae (CPC 18223). a. Colony on MEA; b–h. a series of micro- and macroconidiophores showing conidia in chains. — Scale bars = 10 μm.
Cladosporio australiensis phylogenetice simile, sed hyphis angustioribus, microconidiophoris formantibus et conidiis leniter verruculosis.
Etymology. Named after the host from which it was collected, Phaenocoma prolifera.
Mycelium immersed and superficial, abundant, 1–2.5 μm wide, septate, subhyaline to pale or medium olivaceous-brown, smooth to verruculose, at times forming hyphal ropes. Macroconidiophores macronematous, solitary, arising terminally and laterally from hyphae, erect, slightly flexuous, cylindrical-oblong, 60–100(–200) × 2.5–3 μm, neither geniculate nor nodulose, unbranched or branched below, 2–5-septate, not constricted at septa, pale to medium olivaceous-brown, smooth. Microconidiophores erect, intercalary, subcylindrical, smooth to finely verruculose, pale to medium brown, 0–1-septate, 5–20 × 2–3 μm. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, neither geniculate nor nodulose, 5–20(–25) × (2–)3(–3.5) μm, with 1–4(–6) loci at the apex or 1–3 loci in intercalary cells with loci situated mostly all at more or less the same level, conspicuous, subdenticulate, 1–1.5 μm diam, somewhat thickened and darkened-refractive. Ramoconidia occasionally formed, subcylindrical, 0(–1)-septate, 17–20(–28) × (2–)3(–4) μm. Secondary ramoconidia fusoid-ellipsoid, aseptate, (5–)10–15(–20) × (3–)3.5(–4) μm. Conidia pale to olivaceous-brown, finely verruculose, catenate, in branched chains, branching in all directions, up to 2–4 conidia in the terminal unbranched part of the chain; intercalary conidia ovoid to ellipsoid, aseptate, 4–5(–10) × (2.5–)3(–3.5) μm, with 1–3 distal hila, somewhat thickened, darkened-refractive, 1–1.5 μm diam; small terminal conidia globose, subglobose to obovoid, (3–)4(–5) × 2–3 μm, aseptate, rounded at the apex; microcyclic conidiogenesis not observed.
Culture characteristics — Colonies after 1 wk at 25 °C in the dark, with sparse aerial mycelium and smooth, even margins, reaching 7 cm diam; on OA greenish olivaceous; on MEA dull green (surface and reverse); on PDA grey-olivaceous (surface), and olivaceous-grey in reverse; sporulating profusely on all media.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20529 holotype, cultures ex-type CPC 18221, 18223 = CBS 128769.
Notes — Phylogenetically (Fig. 3), C. phaenocomae is closely allied to C. australiense (described from Eucalyptus in Australia; Bench et al. 2010), but can be distinguished by its narrower hyphae, conidia that are slightly roughened, and the presence of microconidiophores.
Devriesia tardicrescens Crous, sp. nov. — MycoBank MB560019; Fig. 6
Fig. 6.
Devriesia tardicrescens (CPC 18259). a–c. Chlamydospore-like structures formed in culture; d–g. conidiophores with conidia in chains; h. conidia. — Scale bars = 10 μm.
Devriesiae staurosporae similis, sed in cultura tarde crescent et conidiis longioribus.
Etymology. Named after its slow growth rate in culture.
Colonies sporulating on OA. Mycelium consisting of branched, septate, pale brown, smooth, 1.5–2 μm wide hyphae. Conidiophores solitary, erect on creeping hyphae, unbranched or branched, medium brown, smooth, flexuous, 30–200 × 1.5–2.5 μm, 2–11-septate. Conidiogenous cells terminal or lateral, medium brown, subcylindrical, smooth, 15–25 × 1.5–2 μm; proliferating sympodially, scars flattened, thickened, somewhat darkened, 1–1.5 μm wide. Conidia medium brown, smooth, aseptate, subcylindrical to narrowly fusoid-ellipsoidal, apical conidium with obtuse apex, additional conidia with truncate ends, somewhat darkened hila, 0.5–1 μm wide; conidia straight, mostly in branched chains. Ramoconidia with 1–3 apical loci, truncate, subdenticulate, 15–25 × 1.5–2.5 μm. Secondary ramoconidia with 1–2 apical loci, 7–14 × 1.5–2.5 μm. Intercalary and terminal conidia aseptate, (5–)6–7(–8) × (1.5–)2(–2.5) μm. Chlamydospores dark-brown, smooth to verruculose, ellipsoid, 0–1-septate, 10–20 × 7–10 μm; forming additional septa with age, becoming irregular, microsclerotial, up to 25 μm diam.
Culture characteristics — Colonies erumpent, spreading, uneven, with sparse to moderate aerial mycelium, with folded surface and smooth, even, crenate margin. On PDA surface olivaceous-grey, reverse iron-grey; reaching 7 mm diam after 2 wk. On OA surface olivaceous-grey with iron-grey outer margin; reaching 7 mm diam after 2 wk. On MEA surface olivaceous-grey with submerged iron-grey margins, and iron-grey underneath; reaching 10 mm diam after 2 wk.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20530 holotype, cultures ex-type CPC 18259 = CBS 128770.
Notes — Although D. tardicrescens is phylogenetically related to D. staurophora (Fig. 1) and D. shelburniensis (Fig. 1, 2), it has a slower growth rate (10 mm vs > 20–24 mm after 2 wk), and different conidial dimensions than the latter two species (Seifert et al. 2004).
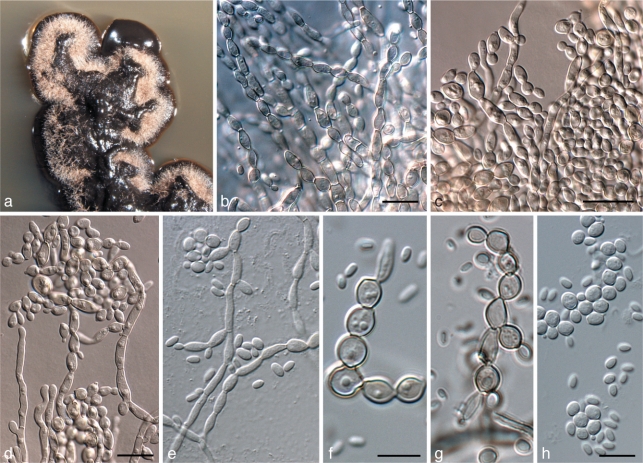
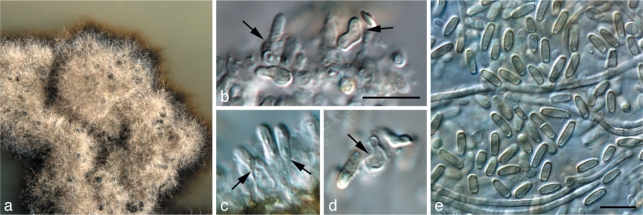
Exophiala capensis Crous, sp. nov. — MycoBank MB560020; Fig. 7
Fig. 7.
Exophiala capensis (CPC 18473). a. Colony on MEA; b, c. Cladophialophora-like state; d–h. Exophiala conidiogenous cells, hyphae and conidia. — Scale bars = 10 μm.
Synanamorph: Cladophialophora sp.
Exophialae bergeri morphologice similis, sed conidiis majoribus, (2–)3–5(–6) × (2–)3–3.5(–4) μm.
Etymology. Named after the Cape Province, where this fungus was collected.
Mycelium consisting of branched, septate, pale brown, smooth, 2–3 μm wide hyphae. Conidiophores dimorphic. Cladophialophora state with erect, solitary conidiophores, up to 80 μm tall. Conidiogenous cells intercalary and terminal, subcylindrical, 8–12 × 2–3 μm, proliferating sympodially. Conidia pale brown, smooth, aseptate to 1-septate, occurring in branched or unbranched chains, ellipsoid to subcylindrical, (7–)8–10(–13) × 2–3(–4) μm, tapering towards truncate ends, 1 μm wide, not thickened nor darkened. Exophiala state. Conidiogenous cells inconspicuous on hyphae, phialidic, lateral or terminal, subcylindrical to ellipsoid, pale brown, smooth, 5–7 × 2–3 μm; phialidic opening inconspicuous, up to 1 μm wide, with small collarette, up to 0.5 μm tall. Conidia pale brown, smooth, ellipsoid to globose, (2–)3–5(–6) × (2–)3–3.5(–4) μm; conidia frequently giving rise to secondary conidia via microcyclic conidiation.
Culture characteristics — Colonies spreading, slightly erumpent, with sparse aerial mycelium and smooth, lobate to regular margins. On MEA surface folded, brown-vinaceous, also in reverse; on potato-dextrose agar irregular with sparse aerial mycelium and even, smooth, crenate margins. On PDA iron-grey in the middle, olivaceous-grey in outer region, iron-grey in reverse. On OA iron-grey, slimy; colonies on all media reaching 7 mm diam after 2 wk.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20531 holotype, cultures ex-type CPC 18473 = CBS 128771.
Notes — Exophiala capensis is phylogenetically (Fig. 1) distant to Exophiala bergeri, and together they appear to represent a different lineage in the Chaetothyriales. For the present, however, we prefer to describe it as a novel species of Exophiala with a Cladophialophora-like synanamorph.
Penidiella aggregata Crous, sp. nov. — MycoBank MB560022; Fig. 8
Fig. 8.
Penidiella aggregata (CPC 18278). a. Colony on MEA; b–g. conidiophores with aggregated conidiogenous loci, and short conidial chains; h. conidia. — Scale bars = 10 μm.
Penidiellae rigidophorae morphologice similis, sed conidiis minoribus, (5–) 6–8 × (2–)2.5(–3) μm.
Etymology. Named after the densely aggregated scars on the conidiogenous cells.
Colonies sporulating on OA. Mycelium consisting of branched, septate, smooth, pale brown, 2–3 μm wide hyphae. Conidiophores solitary, arising from superficial mycelium, erect, brown, smooth, up to 60 μm tall, 3–4 μm wide at base, 3–5-septate, straight to irregularly geniculate-sinuous. Conidiogenous cells terminal, subcylindrical, unbranched, medium brown, 7–20 × 3–3.5 μm, smooth, tapering to a flattened or rounded apical region, scars unthickened, aggregated, somewhat darkened, not refactive, 0.5–1 μm wide. Ramoconidia 0–1-septate, medium brown, smooth, ellipsoidal to obclavate or obovoid, with 1–3 apical hila, 8–15 × 3–4 μm. Intermediate and terminal conidia subcylindrical to ellipsoid, 0(–1)-septate, brown, in chains of up to 6, (5–)6–8 × (2–)2.5(–3) μm; hila truncate, unthickened, somewhat darkened, 0.5–1 μm wide.
Culture characteristics — Colonies spreading, erumpent, with sparse aerial mycelium and even, smooth margins. On PDA surface and reverse iron-grey; reaching 12 mm diam after 2 wk. On OA surface iron-grey; reaching 8 mm diam after 2 wk. On MEA surface folded, iron-grey on surface and reverse; reaching 12 mm diam after 2 wk.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20532 holotype, cultures ex-type CPC 18278 = CBS 128772.
Notes — Penidiella aggregata is morphologically characterised by having apically aggregated, flattened conidial scars on its conidiogenous cells. It is similar to P. rigidophora in its conidial branching patterns (Crous et al. 2007a), but distinct in that conidia are smaller than in P. rigidophora (intercalary and terminal conidia 7–12 × 3–5 μm). Phylogenetically it is related to species of Penidiella and Catenulostroma (Fig. 1, 2).
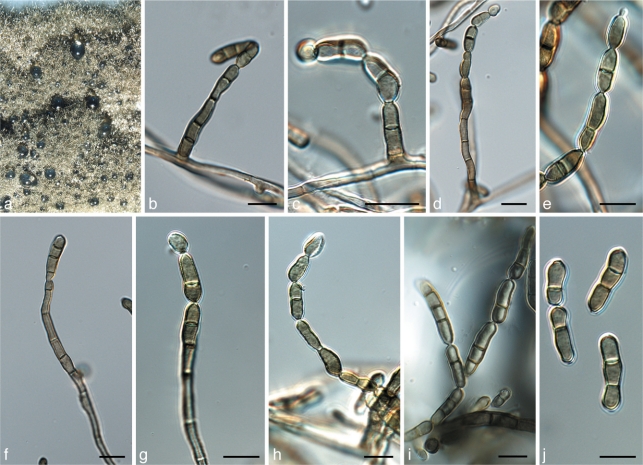
Penidiella ellipsoidea Crous, sp. nov. — MycoBank MB560021; Fig. 9
Fig. 9.
Penidiella ellipsoidea (CPC 18317). a. Colony on PDA; b–i. conidiophores with conidiogenous cells and conidial chains; j. conidia. — Scale bars = 10 μm.
Penidiellae rigidophorae morphologice similis, sed conidiis majoribus, (14–) 20–30(–70) × 4–5(–5.5) μm, 0–7-septatis, saepe in catenis haud ramosis.
Etymology. Named after its typically ellipsoid conidia.
Colonies sporulating on OA. Mycelium consisting of branched, septate, verruculose, medium brown, 3–4 μm wide hyphae. Conidiophores solitary, arising from superficial mycelium, erect, brown, verruculose, up to 90 μm tall, (3–)5–6 μm wide at base, up to 12-septate. Conidiogenous cells terminal, intercalary or lateral, unbranched, brown, 6–15 × 3.5–6 μm, finely verruculose, subcylindrical to somewhat doliiform, tapering to a flattened apical region, scars unthickened, not darkened, nor refactive, 1 μm wide. Conidia medium brown, smooth, subcylindrical to ellipsoid, 0–4(–7)-septate, in mostly unbranched chains of up to 10, (14–)20–30(–70) × 4–5(–5.5) μm; hila truncate, unthickened, not darkened, 1–1.5 μm wide.
Culture characteristics — Colonies spreading, erumpent with sparse aerial mycelium, and smooth, even, crenate margins. On PDA surface folded, iron-grey on surface and reverse; reaching 10 mm diam after 2 wk. On OA surface iron-grey, reaching 12 mm diam. On MEA surface folded, iron-grey on surface and reverse; reaching 10 mm diam after 2 wk.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20533 holotype, cultures ex-type CPC 18318, 18317 = CBS 128773.
Notes — Penidiella ellipsoidea is morphologically similar to P. rigidophora (Crous et al. 2007a), but distinct in that conidia mostly occur in unbranched chains, are larger in size and have more septa than conidia of P. rigidophora (ramoconidia 10–25 × 3–5 μm, 1–3-septate, intercalary and terminal conidia 7–12 × 3–5 μm). Phylogenetically, it is more related to Catenulostroma spp. and Teratosphaeria bellula (Fig. 1, 2).
Teratosphaeria cf. bellula (Crous & M.J. Wingf.) Crous & U. Braun, Stud. Mycol. 58: 10. 2007 — Fig. 10
Fig. 10.
Teratosphaeria cf. bellula (CPC 18281). a. Colony on MEA; b, c. asci with ascospores; d. ascospores; e. ascospores germinating on MEA after 24 h of incubation. — Scale bar = 10 μm.
Basionym. Mycosphaerella bellula Crous & M.J. Wingf., Mycotaxon 46: 20. 1993.
Descriptions — Crous & Wingfield (1993), Taylor & Crous (1998), Crous et al. (2004a, 2008a).
Culture characteristics — Colonies spreading, erumpent, with sparse to moderate aerial mycelium. On PDA surface folded, olivaceous-grey with thin, submerged, iron-grey margin, reverse iron-grey; reaching 8 mm diam after 2 wk. On OA surface folded, olivaceous-grey; reaching 8 mm diam after 2 wk. On MEA surface folded, olivaceous-grey, with thin, submerged, iron-grey margin, reverse iron-grey; reaching 10 mm diam after 2 wk.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CPC 18280, 18281.
Notes — Crous et al. (2008a) designated an epitype for T. bellula, but also revealed this taxon to represent a species complex occurring on several different hosts, characterised by small ascospores with bluntly rounded ends, surrounded by a mucoid sheath. Furthermore, ascospores germinate at right angles to the long axis, darken, and become roughened upon germination. The present isolates from Phaenocoma represent at least two species within this complex (Fig. 1, 2), distinguished morphologically only by lacking a characteristic mucoid sheath. This complex is poorly understood, and hence we have chosen to not name these isolates in the present study, as more gene loci need to be sequenced to resolve their species boundaries.
Teratosphaeria karinae Crous, sp. nov. — MycoBank MB560023; Fig. 11
Fig. 11.
Teratosphaeria karinae (CPC 18255). a, b. Flowers of Phaenocoma prolifera; c. colony on MEA; d, e. hyphal network in leaf bracts with ascomata; f–h. asci with ascospores; i, j. ascospores. — Scale bars = 10 μm.
Teratosphaeriae bellulae similis, sed ascosporis subtiliter guttulatis, sine vagina mucoide, haud fuscatis in statu germinanti et tubis germinationis parallelis ad axem sporae.
Etymology. Named after Karina Louise Crous, who collected the specimen of Phaenocoma prolifera that formed the basis of this study.
Ascomata black, immersed to erumpent, up to 70 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid, straight to slightly curved, 8-spored, 35–45 × 8–10 μm. Ascospores tri- to multiseriate, overlapping, hyaline, finely guttulate, thin-walled, straight, fusoid-ellipsoidal with obtuse ends, widest in the middle of apical cell, not to somewhat constricted at septum, tapering towards both ends, but more prominently towards lower end, (8–)9–10(–11) × (2.5–)3 μm; germinating ascospores on MEA remain hyaline, and germinate from polar ends, with germ tubes parallel to the long axis of the spore.
Culture characteristics — Colonies spreading, erumpent, with moderate aerial mycelium and even, smooth, crenate margins. On PDA surface grey-olivaceous, reverse iron-grey; reaching 13 mm diam after 2 wk. On OA olivaceous-grey, reaching 12 mm after 2 wk. On MEA olivaceous-grey, with a thin, submerged, iron-grey margin, iron-grey underneath; reaching 11 mm diam after 2 wk.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20534 holotype, cultures ex-type CPC 18256, 18255 = CBS 128774.
Notes — The ascospore dimensions of T. karinae are similar to that of T. bellula, which also occurs on this material. However, ascospores of T. karinae are finely guttulate, lack a mucoid sheath (Crous et al. 2008a), and do not darken at germination, with germ tubes being parallel, not 90° to the long axis of the spore, as observed in T. bellula. The T. bellula complex contains many unresolved cryptic species, but more genes would have to be sequenced to completely resolve their species boundaries (Fig. 1, 2).
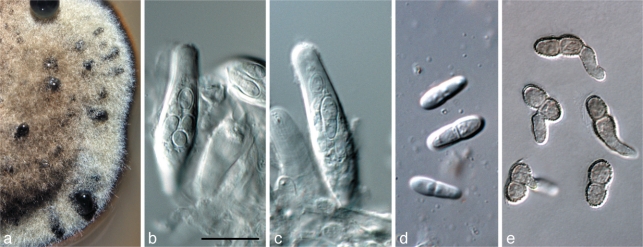
Toxicocladosporium pseudoveloxum Crous, sp. nov. — MycoBank MB560024; Fig. 12
Fig. 12.
Toxicocladosporium pseudoveloxum (CPC 18257). a. Colony on PDA; b–h. conidiophores with conidiogenous cells and conidial chains. — Scale bars = 10 μm.
Toxicocladosporio veloxo simile, sed ramoconidiis brevioribus, 8–15 × 2.5–4 μm.
Etymology. Named after its morphological similarity to T. veloxum.
Mycelium on SNA consisting of branched, septate, pale brown, smooth, 1.5–2 μm wide hyphae. Conidiophores solitary, macronematous, subcylindrical, straight to geniculous-sinuous, or irregularly curved, unbranched or branched above, 2–5-septate, dark brown, finely verruculose, walls thick, septa dark-brown, 20–50 × 3–4 μm. Conidiogenous cells integrated, terminal or lateral, subcylindrical with slight taper towards apex, 10–15 × 3–4 μm; proliferating sympodially with 1–3 apical loci, 1–1.5 μm wide, thickened, darkened and refractive. Conidia catenate in branched chains, medium to dark brown, thick-walled, with dark, thick septa, smooth; ramoconidia 0–1-septate, broadly ellipsoid to subcylindrical, 8–15 × 2.5–4 μm; intermediate and terminal conidia ellipsoid, pale to medium brown, aseptate, (6–)7–10(–11) × (2–)2.5(–3) μm; hila protruding, 0.5–1.5 μm wide, thickened, darkened and refractive.
Culture characteristics — Colonies spreading with moderate aerial mycelium and even, smooth margins. On PDA reaching 20 mm diam after 2 wk, olivaceous-grey on surface and reverse. On OA surface olivaceous-grey, reaching 20 mm diam. On MEA surface olivaceous-grey, somewhat folded; reverse iron-grey, reaching 25 mm diam.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20535 holotype, cultures ex-type CPC 18257 = CBS 128775, 18274, 18275, 18471 = CBS 128777.
Notes — Phylogenetically (Fig. 1) and morphologically T. pseudoveloxum is similar to T. veloxum and other Toxicocladosporium spp. (Crous et al. 2009d), but differs in that it has shorter ramoconidia (8–15 × 2.5–4 μm), than T. veloxum (15–18 × 2.5–4 μm). Blast searches using the ITS sequence revealed high identity to T. protearum (GenBank HQ599586; Identities = 647/653 (99 %), Gaps = 3/653 (0 %)), T. veloxum (GenBank FJ790288; Identities = 607/613 (99 %), Gaps = 4/613 (0 %)), T. chlamydosporum (GenBank FJ790284; Identities = 604/615 (99 %), Gaps = 6/615 (0 %)) and T. banksiae (GenBank HQ599598; Identities = 648/663 (98 %), Gaps = 7/663 (1 %)).
Xenophacidiella Crous, gen. nov. — MycoBank MB560056
Phacidiellae morphologice similis, sed conidiomatibus pycnidialibus, conidiis pigmentis, haud hyalinis, binis in catenis falsis, disarticulantibus.
Type species. Xenophacidiella pseudocatenata Crous.
Etymology. Not Phacidiella, which it resembles morphologically.
Mycelium consisting of branched, septate, brown, verruculose, 1.5–2 μm wide hyphae. Conidiomata eustromatic, pycnidial, multilocular, with several ostioles, erumpent, grey-brown; wall of 2–3 layers of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity of conidioma, embedded in mucoid layer, subcylindrical, hyaline, smooth, proliferating inconspicuously percurrently at truncate apex. Conidia medium brown, verruculose, subcylindrical, apex obtusely rounded, base truncate, thin-walled, aseptate, straight to slightly curved, at times occurring in chains of two.
Xenophacidiella pseudocatenata Crous, sp. nov. — MycoBank MB560025; Fig. 13
Fig. 13.
Xenophacidiella pseudocatenata (CPC 18472). a. Colony on MEA with conidiomata exuding black spore masses; b–d. conidiogenous cells (arrows) giving rise to conidia; e. brown, verruculose, cylindrical conidia that disarticulate at the median septum. — Scale bars = 10 μm.
Conidiomata eustromatica, pycnidiales, multiloculares, usque ad 120 μm diam, pariete ex 2–3 stratis texturae angularis. Cellulae conidiogenae subcylindraceae, hyalinae, leves, 4–7 × 2.5–3.5 μm, ad apicem inconspicue percurrente proliferantes. Conidia modice brunnea, verruculosa, subcylindrica, apice obtuse rotundato, basi truncate, tenuitunicata, aseptata, interdum bina in catenis falsis, disarticulantibus, (4–)5–7(–8) × 2(–2.5) μm.
Etymology. Named after the conidia that become septate, separating into two cells, creating the impression of false chains.
Mycelium consisting of branched, septate, brown, verruculose, 1.5–2 μm wide hyphae. Conidiomata eustromatic, pycnidial, multilocular, with several ostioles, erumpent, grey-brown on OA, up to 120 μm diam; wall of 2–3 layers of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity of conidioma, embedded in mucoid layer, subcylindrical, hyaline, smooth, 4–7 × 2.5–3.5 μm, proliferating inconspicuously percurrently at truncate apex. Conidia medium brown, verruculose, subcylindrical, apex obtusely rounded, base truncate, thin-walled, aseptate, straight to slightly curved, at times occurring in chains of two, (4–)5–7(–8) × 2(–2.5) μm.
Culture characteristics — Colonies spreading, erumpent, irregular with sparse aerial mycelium and even, smooth, crenate margins. On PDA with folded surface, olivaceous-grey; reverse iron-grey, reaching 8 mm diam after 2 wk. On OA olivaceous-grey, not folded, smooth, reaching 10 mm. On MEA surface folded, olivaceous-grey; reverse iron-grey, reaching 8 mm diam.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38" E 19°16′9.7", on leaf bracts of Phaenocoma prolifera, 2 May 2010, K.L. Crous & P.W. Crous, CBS H-20536 holotype, cultures ex-type CPC 18472 = CBS 128776, CPC 18279.
Notes — The genus Xenophacidiella resembles Phacidiella in having disarticulating chains of conidia. Phacidiella is distinct, however, in having acervular conidiomata, and hyaline, smooth, aseptate, subcylindrical conidia (Sutton 1980). The recently described P. eucalypti (Crous et al. 2007b), which probably represents yet another genus in this complex, is also phylogenetically distinct from X. pseudocatenata, being associated with Ostropales whereas Xenophacidiella is associated with Capnodiales. Based on the LSU and ITS phylogenies (Fig. 1, 2), the closest sister taxa are Phaeothecoidea protea and Penidiella spp.
DISCUSSION
Knowing the number of species that exist on earth is fundamental to understanding and protecting the world’s biodiversity, and thus estimating the number of fungal species has been discussed for a great number of years (Fries 1825, Bisby & Ainsworth 1943, Pirozynski 1972, Pascoe 1990, Hawksworth 1991, 1998, 2001, 2004, Dreyfuss & Chapela 1994, Rossman 1994, Hyde et al. 1997, Fröhlich & Hyde 1999, Crous et al. 2006b). In spite of this, the number that is commonly used to argue for fungal biodiversity is the 1.5 M estimate by Hawksworth (1991), though the fungal biodiversity in the Southern Hemisphere seems to greatly exceed this estimate (Crous et al. 2006b, Marincowitz et al. 2008a, b). Furthermore, recent 454 pyrosequencing DNA-based techniques like those employed by Buée et al. (2009) showed an unexpected high level of novel fungal biodiversity in forest soils, suggesting that former specimen-based estimates were far too conservative.
Without taking species occurring in soil and on insects (Suh et al. 2005) into account, Crous et al. (2006b) estimated that at least 200 000 unique fungal species should occur in southern Africa. In Australia, however, Pascoe (1990) estimated that there could be at least ten times as many fungi as vascular plants. Researchers working in specific niches, tended to have much higher estimates, namely 1 M on tropical plants (Smith & Waller 1992), 1.3 M endophytic fungi (Dreyfuss & Chapela 1994), or a ratio of 33 : 1 fungi per plant species for palm fungi (Fröhlich & Hyde 1999). Hyde et al. (1997) also reported that 75 % of all fungi collected on palms were new to science, followed by Marincowitz et al. (2008b) who reported 43 % of the taxa collected from Proteaceae leaf and twig litter to be undescribed, while Crous et al. (2009d) described eight unique species from a single leaf spot of a eucalypt tree growing in Madagascar.
The fungal biodiversity in South Africa has been poorly studied to date, and no species have thus far been described from Phaenocoma prolifera. Using the same damp chamber technique as employed here, Crous et al. (1996) described four unique hyphomycetes from Podocarpus elongatus, and five from Syzygium cordatum (Crous et al. 1995), while later studies added at least eight more species from this host (Sutton & Crous 1997, Pavlic et al. 2004, 2009), with several more host-specific fungi awaiting description.
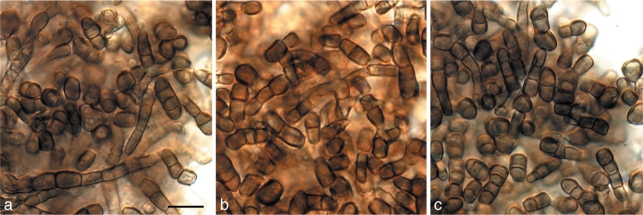
In spite of the new species described in this study, several other taxa were also isolated that have known, wider host ranges. These include Teratosphaeria bellula (ex-type strain of species: CBS 111700; cryptic species isolated here CPC 18280, 18281) and Batcheloromyces leucadendri (ex-type strain of species: CBS 111577; isolated here CPC 18277; Fig. 14), both representing well-known pathogens of Proteaceae (Crous et al. 2004a, 2008a), which may be moving among different substrates in search of their ideal hosts (e.g. pogostick hypothesis; Crous & Groenewald 2005). Presumed saprobic species included two species of Penicillium, namely P. crocicola (= P. thomii) (DTO 132D6 = DTO 133G5) and P. ramulosum (DTO 133G6 = DTO 133G7) (Visagie et al. 2009), and three species of Cladosporium, namely C. cladosporioides (CPC 18230), C. perangustum (CPC 18228, 18229), and C. ramotenellum (CPC 18224) (Bensch et al. 2010).
Fig. 14.
Batcheloromyces leucadendri (CPC 18277). a–c. Colony sporulating in vitro on OA, showing disarticulating conidial chains. — Scale bar = 10 μm.
The present study revealed 17 cultivatable species of microfungi to occur on 10 leaf bracts of Phaenocoma prolifera, of which nine of the 11 observed novelties could be named. For several taxa only a few isolates were recovered, meaning that the full variation present in populations of these species is not known at present. Furthermore, based on the isolation technique employed here, mostly hyphal ascomycetes (forming ascomata, or sporulating hyphomycetes or coelomycetes) were recovered, leaving out many other fungi that are certainly also present. Undoubtedly, if novel sequencing techniques such as 454 pyrosequencing technology were to be employed, a large portion of uncultivatable and largely unseen fungi would also be detected, which would greatly increase this number. In contrast to the statement of Hawksworth (1991) that each plant species can be expected to have 5–6 novel species of fungi, using these novel techniques one should be able to refine the question as to how many novel species could be expected per different plant part. Recent work by Batzer and colleagues dealing with flyspeck and sooty blotch of apples, for instance, have shown the epiphytes on apple fruit surfaces to be different from fungi occurring on leaves and branches of this host (Batzer et al. 2008, Yang et al. 2010).
Based on this initial look at microfungi present in a single flower of one plant species in southern Africa, as well as the observations from southern Africa discussed in Crous et al. (2006b), it seems that the world estimate of 1.5 M (based on 5–6 novel fungal species) is too conservative. However, as this was a senescent flower, and the host specificity of most of the taxa treated remains unknown, it is premature to draw definitive conclusions about species numbers based on these data, as the everlasting flowers could simply act as catch crops for fungi with wider host ranges. This is certainly true for the majority of other taxa discussed here, of which we only suspect members of Penidiella, Teratosphaeria and Xenophacidiella to be host specific based on currently published data. Further in-depth studies involving more hosts, different plant parts, and different growth stages are now required, to see if this trend also holds true for other plant species from the Cape Floral Region.
Acknowledgments
Prof. dr U. Braun (Martin-Luther-Univ., Halle, Germany) is thanked for providing the Latin diagnoses. The authors thank the technical staff, A. van Iperen (cultures), M. Vermaas (photo plates), and M. Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Batzer JC, Mercedes Diaz Arias M, Harrington TC, Gleason ML, Groenewald JZ, Crous PW.2008. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 100: 246 – 258 [DOI] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, et al. 2010. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology 67: 1 – 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby GR, Ainsworth GC.1943. The numbers of fungi. Transactions of the British Mycological Society 26: 16 – 19 [Google Scholar]
- Bond P, Goldblatt P.1984. Plants of the Cape flora: A descriptive catalogue. Journal of South African Botany (Supp.) 13: 1 – 455 [Google Scholar]
- Buée M, Reich M, Murat C, Morin E, Nilsson RH, et al. 2009. 454 pyrosequencing analyses of forest soils reveal an unexpected high fungal diversity. New Phytologist 184: 449 – 456 [DOI] [PubMed] [Google Scholar]
- Cowling RM, Costanza R, Higgins SI.1997. Services supplied by South African fynbos ecosystems. In: Dailey GC. (ed), Nature’s services: societal dependence on natural ecosystems: 345 – 362 Island Press, USA: [Google Scholar]
- Crous PW.1998. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1 – 170 APS Press, Minnesota, St. Paul, USA: [Google Scholar]
- Crous PW.2002. Adhering to good cultural practice (GCP). Mycological Research 106: 1378 – 1379 [Google Scholar]
- Crous PW, Braun U, Groenewald JZ.2007a. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1 – 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME.2004a. Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1 – 228 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G.2004b. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 [Google Scholar]
- Crous PW, Groenewald JZ.2005. Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463 – 470 [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD.2006a. Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology 55: 213 – 226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ.2009a. Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22: 38 – 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Mohammed C, Glen M, Verkley GJM, Groenewald JZ.2007b. Eucalyptus microfungi known from culture. 3. Eucasphaeria and Sympoventuria genera nova, and new species of Furcaspora, Harknessia, Heteroconium and Phacidiella. Fungal Diversity 25: 19 – 36 [Google Scholar]
- Crous PW, Rong IH, Wood A, Lee S, Glen H, et al. 2006b. How many species of fungi are there at the tip of Africa? Studies in Mycology 55: 13 – 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al. 2009b. Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17 – 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Seifert KA, Castañeda Ruiz RF.1996. Microfungi associated with Podocarpus leaf litter in South Africa. South African Journal of Botany 62: 89 – 98 [Google Scholar]
- Crous PW, Summerell BA, Mostert L, Groenewald JZ.2008a. Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia 20: 59 – 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkleij GJM, Groenewald JZ, Samson RA. (eds). 2009c. Fungal Biodiversity. CBS Laboratory Manual Series. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Crous PW, Wingfield MJ.1993. Additions to Mycosphaerella in the fynbos biome. Mycotaxon 46: 19 – 26 [Google Scholar]
- Crous PW, Wingfield MJ, Groenewald JZ.2009d. Niche sharing reflects a poorly understood biodiversity phenomenon. Persoonia 22: 83 – 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Kendrick WB.1995. Foliicolous dematiaceous hyphomycetes from Syzygium cordatum. Canadian Journal of Botany 73: 224 – 234 [Google Scholar]
- Crous PW, Wingfield MJ, Park RF.1991. Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628 – 632 [Google Scholar]
- Crous PW, Wood AR, Okada G, Groenewald JZ.2008b. Foliicolous microfungi occurring on Encephalartos. Persoonia 21: 135 – 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Midgley GF, Hoffman MT.1994. Linking biodiversity to ecosystem function: a challenge to fynbos ecology. South African Journal of Science 90: 319 – 321 [Google Scholar]
- Dreyfuss MM, Chapela IH.1994. Potential of fungi in the discovery of novel, low-molecular weight pharmaceuticals. In: Gullo VP. (ed), The discovery of natural products with therapeutic potential: 49 – 80 Butterworth-Heinemann, London, UK: [DOI] [PubMed] [Google Scholar]
- Fries EM.1825. Systema Orbis Vegetabilis. Vol. 1 Typographia Academica, Lund: [Google Scholar]
- Fröhlich J, Hyde KD.1999. Biodiversity of palm fungi in the tropics: are global fungal diversity estimates realistic? Biodiversity and Conservation 8: 977 – 1004 [Google Scholar]
- Goldblatt P.1997. Floristic diversity in the Cape Flora of South Africa. Biodiversity and Conservation 6: 359 – 377 [Google Scholar]
- Hawksworth DL.1991. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycological Research 95: 641 – 655 [Google Scholar]
- Hawksworth DL.1998. The consequences of plant extinctions for their dependant biotas: an overlooked aspect of conservation science. In: Peng CI, Lowry PP. (eds), Rare, threatened, and endangered floras of Asia and the Pacific Rim: 1 – 15 Institute of Botany, Academia Sinica, Taipei, China: [Google Scholar]
- Hawksworth DL.2001. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycological Research 105: 1422 – 1432 [Google Scholar]
- Hawksworth DL.2004. Fungal diversity and its implications for genetic resource collections. Studies in Mycology 50: 9 – 18 [Google Scholar]
- Hilton-Taylor C.1996. Red data list of southern African plants. National Botanical Institute, Pretoria, South Africa: [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG.1998. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183 – 189 [DOI] [PubMed] [Google Scholar]
- Hyde KD, Fröhlich J, Taylor JE.1997. Diversity of ascomycetes on palms in the tropics. In: Hyde KD. (ed), Biodiversity of tropical microfungi: 141 – 156 Hong Kong University Press, Hong Kong: [Google Scholar]
- Jackson WPU.1990. Origins and meanings of names of South African plant genera. UCT Ecolab, c/o Botany Department, Rondebosch: [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T.2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059 – 3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekemoer M.2002. Systematics of the Relhaniina (Asteraceae - Gnaphalieae). Unpublished PhD thesis, Rand Afrikaans University, South Africa: [Google Scholar]
- Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ.2008a. Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series 7: 1 – 166 [Google Scholar]
- Marincowitz S, Groenewald JZ, Wingfield MJ, Crous PW.2008b. Species of Botryosphaeriaceae occurring on Proteaceae. Persoonia 21: 111 – 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe IG.1990. History of systematic mycology in Australia. In: Short PS. (ed), History of systematic botany in Australia: 259 – 264 Australian Systematic Botany Society, South Yarra, Australia: [Google Scholar]
- Pavlic D, Slippers B, Coutinho TA, Gryzenhout M, Wingfield MJ.2004. Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Studies in Mycology 50: 313 – 322 [Google Scholar]
- Pavlic D, Slippers B, Coutinho TA, Wingfield MJ.2009. Molecular and phenotypic characterisation of three phylogenetic species discovered within the Neofusicoccum parvum / N. ribis complex. Mycologia 101: 636 – 647 [DOI] [PubMed] [Google Scholar]
- Pirozynski KA.1972. Microfungi of Tanzania. Mycological Papers 129: 1 – 64 [Google Scholar]
- Rayner RW.1970. A mycological colour chart. Commonwealth Mycological Institute, Kew, UK: [Google Scholar]
- Rossman AY.1994. A strategy for an all-taxa inventory of fungal diversity. In: Peng CI, Chen CH. (eds), Biodiversity and terrestrial ecosystems: 169 – 194 Institute of Botany, Academia Sinica, Taipei, China: [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink MS, et al. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105 – 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert KA, Nickerson NL, Corlett M, Jackson ED, Louis-Seize G, Davies RJ.2004. Devriesia, a new hyphomycete genus to accommodate heat-resistant, cladosporium-like fungi. Canadian Journal of Botany 82: 914 – 926 [Google Scholar]
- Smith D, Waller JM.1992. Culture collections and microorganisms: their importance in tropical plant pathology. Fitopathologia Brasileira 17: 1 – 8 [Google Scholar]
- Suh S-O, McHugh JV, Pollock DD, Blackwell M.2005. The beetle gut: a hyperdiverse source of novel yeasts. Mycological Research 109: 261 – 265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton BC.1980. The coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew, UK: [Google Scholar]
- Sutton BC, Crous PW.1997. Lecanostictopsis gen. nov. and similar fungi from Syzygium species. Mycological Research 101: 215 – 225 [Google Scholar]
- Swofford DL.2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts, USA: [Google Scholar]
- Taylor HC.1977. The Cape floral kingdom. An ecological view. Proceedings of the Second National Weeds conference of South Africa: 19 – 33 [Google Scholar]
- Taylor JE, Crous PW.1998. Mycosphaerella bellula. IMI Descriptions of Fungi and Bacteria, no. 1345.
- Vilgalys R, Hester M.1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238 – 4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Roets F, Jacobs K.2009. A new species of Penicillium, P. ramulosum sp. nov., from the natural environment. Mycologia 101: 888 – 895 [DOI] [PubMed] [Google Scholar]
- Wessels J, Anandajayasekeram P, Littlejohn G, Martella D, Marasas C, Coetzee C.1997. Socioeconomic impact of the Proteaceae development and transfer program. Southern African Center for Cooperation in Agricultural and Natural Resources Research & Training, Gaborone, Botswana: [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J.1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315 – 322 Academic Press, San Diego, California, USA: [Google Scholar]
- Yang HL, Sun GY, Batzer JC, Crous PW, Groenewald JZ, Gleason ML.2010. Novel fungal genera and species associated with the sooty blotch and flyspeck complex on apple in China and the USA. Persoonia 24: 29 – 37 [DOI] [PMC free article] [PubMed] [Google Scholar]