Abstract
Survival rates of individuals with non-Hodgkin lymphoma (NHL) have increased in the past several years, as has the prevalence of older adults who are managing late and long-term effects of the disease and its treatment. In this integrative review, the state of the science for determining the quality of life (QOL) among NHL survivors is outlined. An online search of Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and the Cochrane Library databases was conducted using the following Keywords: non-Hodgkin lymphoma, health-related quality of life, quality of life, and impact of cancer. Eighteen studies published between 2000 and 2010 are reviewed. Of these, 17 were descriptive, cross-sectional designs, and one was a systematic review. The studies included participants of varying ages and years post-diagnosis as reported in several countries. Importantly, many used one or more QOL measures as outcome variables. Future research is needed on older and minority cancer populations and should include longitudinal and interventional studies.
Keywords: Quality of life, Non-Hodgkin lymphoma, survivors, survivorship, older adult
Introduction
Non-Hodgkin lymphoma (NHL) is the sixth most common cancer in the United States, with more than 65 540 new NHL diagnoses and 20 210 deaths expected in 2010 [1]. Incidence and prevalence rates increase with age, and more than 70% of NHLs are diagnosed after 55 years of age [1] and the overall 5-year survival is 68% [2]. Belonging to a group of hematologic cancers, NHL affects the white cells within the lymphatic system. Lymphoma can be slow-growing (indolent) or aggressive (fast-growing). Individuals with human immunodeficiency virus (HIV)-associated NHL are more likely to have high-grade histology and respond poorly to treatment [3]. In addition, NHL is a chronic illness with fluctuating remissions and exacerbations with varying symptoms: fevers, fatigue, weight loss, night sweats, and even localized pain dependent on the involvement of the tumors [4]. In fact, many survivors live with the disease for years and are often diagnosed during routine examinations [5].
In general, cancer is no longer synonymous with a death sentence; some types are viewed as manageable, chronic conditions, as is frequently the case with NHL. This is reflected in the change of terminology associated with cancer care; for example, individuals with cancer are no longer perceived as victims but instead as survivors who live for years following a cancer diagnosis [6,7]. While survivorship is often celebrated, cancer has a significant impact on survivors in terms of long-term health and psychosocial sequelae [6,8–11]. For example, cancer survivors are at increased risk for developing secondary malignancies and other diseases (e.g. cardiovascular disease, diabetes, osteoporosis) [8,9,11,12]. In addition, Hewitt et al. [13] reported that cancer survivors have an almost two-fold greater likelihood of having at least one functional limitation.
The presence of one or more coexisting conditions or ailments in addition to a primary disease such as cancer is known as a comorbidity [14,15]. When a survivor has another comorbid condition, they may experience a decline in functional status that can negatively influence their quality of life (QOL). These comorbid conditions become more prevalent as people age, complicating the illness trajectory of NHL. However, the psychosocial and QOL needs of older adults with NHL remain understudied [4]. Because few data exist on older NHL survivors and the effects of various treatments on their QOL, it is important to understand their QOL domains in order to better address their needs. The purpose of this article is to provide an integrative review that reports on the science for determining QOL among older NHL survivors. The review is focused on QOL measures and treatment effects in the identified studies.
Methods
Articles were retrieved for review via a combination of computer and manual searches of selected QOL and cancer-related publications. A comprehensive, online database search of Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and the Cochrane Library was conducted for NHL research articles published between January 2000 and April 2010. The following search terms were used alone and in combination: non-Hodgkin lymphoma, health-related quality of life, quality of life, and impact of cancer.
Since there is a paucity of data regarding the QOL of older NHL survivors, studies that included younger NHL survivors and those with subsamples of NHL survivors were used in this analysis. However, there was a lack of clarity in age delineation in several studies, but it is assumed that individuals aged ≥65 years are older adults per Medicare requirements, unless explicitly stated otherwise [16]. Except for Mols et al. [16], no studies delineated age.
Articles focused on central nervous system and T-cell cutaneous lymphomas were excluded because their treatment and clinical courses differ from other NHL subtypes [17]. Additionally, publications lacking a standardized QOL-related outcome measure were excluded. Articles were retrieved in the English language, and publication types were limited to primary research reports and systematic reviews. Editorials, opinions, and case studies were excluded.
Articles were reviewed initially by abstracts and titles, yielding 42 abstracts from Medline, 143 abstracts from CINAHL, 12 abstracts from PsycINFO, and no abstracts from the Cochrane Library. Duplicate articles were excluded, as were abstracts that did not meet the inclusion criteria. The full texts of the remaining 98 articles were read. Eighteen articles met the inclusion criteria and were selected for review. Each article was critiqued and appraised for the quality of the research evidence in relationship to QOL for NHL survivors (Figure 1).
Figure 1.
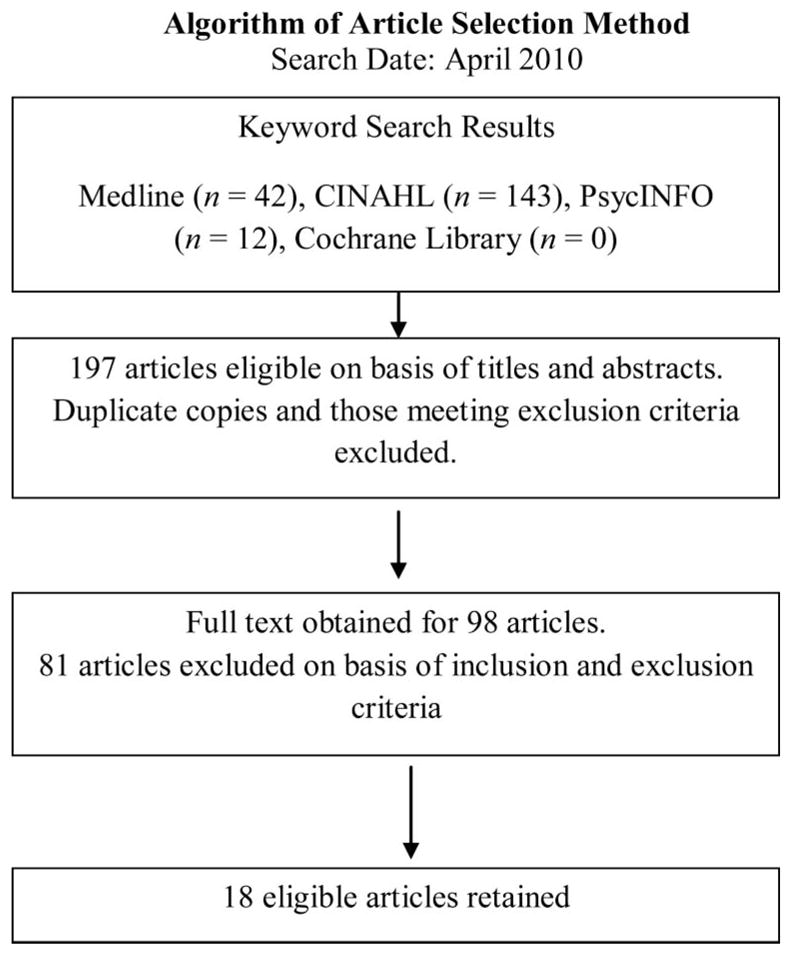
Algorithm of the process used to search for and select appropriate articles to review for indicators of the quality of life of non-Hodgkin lymphoma survivors. CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Results of the literature review
General characteristics
The majority (15/18) of the selected studies were published within the past 5 years. Twelve of the studies were based in the USA, which has a larger cancer database and registry than those in the countries of the remaining studies: The Netherlands (n =2), Canada (n =1), Israel (n =1), and the United Kingdom (n =1). Seventeen of the studies were descriptive, used a cross-sectional design with varying age ranges, and reported on the number of years post-diagnosis. Sociodemographic characteristics were homogeneous across studies.
Quality of life measures
It is generally agreed that QOL is a subjective, multidimensional construct with both positive and negative aspects [18]. QOL domains include physical, psychological, social, functional, and spiritual/existential; each will be addressed. Instruments vary, with the physical well-being domain included most often; however, no QOL instrument is accepted consistently as a gold standard [19]. As shown in Table I, half of the studies (n =9) used more than one QOL outcome measure. Additional instruments that are not included in Table I are the Functional Assessment of HIV Infection [20], Medical Outcomes Study-Social Support [21], Life Impact [22], PTSD Inventory [23], and PTSD Checklist-Civilian Version [24].
Table I.
General characteristics of reviewed studies
| Authors | Design | Purpose | Sample characteristics | QOL-related measures | Findings | Strengths and limitations |
|---|---|---|---|---|---|---|
| Ahles et al. (2005) [49] | Cross-sectional, New Hampshire, USA | Compare QOL of breast and lymphoma svrs treated with standard- dose, systemic CT or local surgery/RT | N =103 NHL svrs, mean age 55.8 years (SD13.5); chemotherapy(n =66), mean age 50.4 years (SD 12.8); local therapy (n =37) | QOL-CS | Svrs treated with systemic CT (n =66) scored significantly lower on overall QOL compared with svrs treated with surgery and/or RT (p =0.04, n = 37), lymphoma svrs treated with chemotherapy scored worse on the physical subscale than those treated with surgery and/or RT (p =0.01) | Strengths: appropriate instruments used to capture long-term effects. Limitations: recruitment from one site, little diversity in sample, small number of lymphoma svrs, not randomly assigned to treatments, no conceptual framework |
| Arden-Close et al. (2010) [50] | Systematic review | (1) Identify and evaluate commonly used HRQOL measures; (2) compare HRQOL in pts with NHL with general population; (3) assess association between HRQOL and different treatments, demographics, medical and psychological variables | 18 identified eligible studies | SF-36, EORTC QLQ C-30, FACT-An, CARES, QOL-CS | 18 studies included adult svrs with lymphoma with histological diagnoses from various countries, and various ages post- diagnosis | Strengths: nine studies were longitudinal or case controlled, focus on lymphoma svrs. Limitations: cross- sectional designs, lack of demographic reporting of means and standard deviations, small samples, no conceptual framework |
| Arora et al. (2007) [45] | Cross-sectional, Los Angeles SEER registry | Address conducting population-based survivorship research using cancer registries | N = 408 NHL svrs, mean age 59.7 years (SD 15), 2–5 years post- treatment; CT: 50.2%, CT + RT: 33.3%, BMT/SCT: 9.6% | SF-36 | Older adults 65+ (19.9%) less likely to be lost to follow-up than younger adults 20–44 years old (37.6%), p <0.01 | Strengths: SEER registry for largest cohort of multiple cancers, use of Andersen’s Behavioral Model for Health Services Utilization and Wilson & Cleary’s HRQOL Model conceptual framework. Limitations: cross- sectional, subject burden with 52-page questionnaire |
| Bellizzi et al. (2007) [22] | Cross-sectional, Los Angeles County Cancer Surveillance Program | Examine positive and negative life changes of NHL svrs and effects on physical and mental function | N = 308 NHL svrs, median age 59.8 years (SD 14.9), 3.5 years post- treatment; 94.2% had CT as part of treatment | SF-36, Life Impact Scale that identifies positive and negative changes, Life Orientation Test- Revised | Positive life change increased with physical functioning when examining overall change (p = 0.01) and health behaviors (p = 0.01); NHL had positive effect on relationships, but negative effect on finances | Strengths: first to examine positive and negative life changes. Limitations: cross-sectional, psychometrics of Life Impact Scale, lack of diverse sample demographically and in disease-related characteristics, no conceptual framework |
| Bellizzi et al. (2009) [31] | Cross-sectional, California | Examine correlates of physical activity and QOL in NHL svrs | N = 319 NHL svrs, mean age 59.8 years (SD 14.8), 2–6 years post- treatment; CT: 48.9%, CT + RT: 33.9%, BMT/SCT: 10.7% | SF-36 | 25% svrs met 150 mins+ goal of exercise per week, 53% reported some activity, 20% reported no activity; NHL svrs who engaged in some form of physical activity had better mental and physical health, improved QOL, p <0.001 | Strengths: first to describe exercise behavior in NHL svrs. Limitations: cross-sectional, self- report of physical activity, no conceptual framework |
| Crespi et al. (2010) [51] | Cross-sectional, NC registry- based sample | (1) Examine reliability and validity of the IOCv2 scales’ measurement comparing breast and NHL svrs; (2) compare results between survivor groups and evaluate generalizability of the IOCv2 | N = 652 NHL svrs, mean age 51.9 (SD 14.2), 2–44 years post-diagnosis; treatments not discussed | IOC, FACT-Lym, SF-36 | Reliability and validity support associations of the scale, breast and NHL svrs have similar IOC domains, scale may be generalizable to long- term svrs but needs to be tested with other cancer svrs | Strengths: use of 47-item IOC vs. 81-item instrument, large NHL sample. Limitations: cross-sectional, less diverse sample, no conceptual framework |
| Diamond et al. (2010) [20] | Cross-sectional; Orange and San Diego counties, California | Compare QOL characteristics and survival of pts with NHL with and without HIV infection | N = 50 NHL svrs without HIV, median age 45 years for entire sample (range 17–70 years), 1 year post-diagnosis; CT: 84% | FACT-G, FAHI | HIV+ NHL pts had worse overall QOL and survival than HIV− NHL pts, p <0.0001, emotional well-being did not statistically differ between HIV+ and HIV− NHL svrs | Strengths: NHL subgroups with and without HIV for comparison. Limitations: cross- sectional, small NHL sample, few women, no conceptual framework |
| Geffen et al. (2003) [23] | Cross-sectional, Israel | Explore relationship between PTSD and HRQOL | N = 44 cancer svrs, n = 36 NHL svrs, median age 51 years (range 27–80), 2 years post-treatment; CT: 54%, RT: 14%, CT +RT: 32% | SF-36, PTSD Inventory | Lymphoma svrs had lower physical HRQOL than controls, p <0.05; intrusion scale and avoidance scale (p <0.01) associated with intense symptoms at early disease onset | Strengths: comparison group, PTSD and HRQOL correlation. Limitations: cross- sectional, small NHL sample, no conceptual framework |
| Mols et al. (2007) [41] | Cross-sectional survey, Eindhoven Cancer Registry, The Netherlands | Explore long-term effects of NHL and treatment on HRQOL | N = 221 NHL svrs, mean age at survey 55.3 years; CT: 37%, RT: 15%, CT + RT: 26%, surgery + RT + CT: 13%, watchful waiting: 9%; 5–15 years post- diagnosis | SF-36, QOL-CS | NHL svrs who had CT experienced worse psychological and social well-being than those who did not have CT, chronic conditions associated with poorer physical functioning, more pain, those employed reported more vitality and better mental well-being scores | Strengths: diverse population treated throughout The Netherlands, large sample, initial response rate (80%). Limitations: cross-sectional, follow- up was difficult due to low response rate (unverifiable addresses), no conceptual framework |
| Mols et al. (2007) [16] | Cross-sectional, Eindhoven Cancer Registry, The Netherlands | Compare HRQOL and healthcare utilization of long-term cancer svrs using population-based study comparing svrs over and under 70 years of age | N = 294 NHL svrs, median age 53 years for NHL svrs < 70, median age 76 years for NHL svrs >70, 5–15 years post- diagnosis; NHL svrs, surgery: n = 28, RT: n = 102, CT: n = 160, watchful waiting: n = 16 | SF-36 | NHL svrs had lower scores for general health perception and vitality compared to normative population (p <0.001); older age, ≥1 comorbid disease, lower educational level, and current occupation had poorer HRQOL (p < 0.01); >70-year- old NHL svrs visited their oncologist more than their PCP when compared to < 70 years, p < 0.01 | Strengths: work changes/patterns exist in long- term svrs; work change correlated with HRQOL. Limitations: cross-sectional, limited follow-up, no conceptual framework |
| Pettengell et al. (2008) [43] | Cross-sectional, United Kingdom | Explore relationship between disease activity and health functioning on HRQOL | N = 222 follicular lymphoma, median age 60.4 years (SD 10.6) | FACT-Lym | Svrs who relapsed had worse QOL and physical and mental functioning compared to newly diagnosed, in partial or complete remission, or disease-free svrs | Strengths: health outcomes reported at different cancer stages, large sample. Limitations: subgroups were small, cross-sectional, no conceptual framework |
| Reeve et al. (2009) [32] | Cross-sectional, SEER data and MHOS, 1998– 2003 | Quantify extent of HRQOL changes before and after cancer diagnosis | N = 1432 prostate, breast, colorectal, lung, bladder, endometrial, kidney, melanoma, and NHL svrs, mean age 73.86 (SD 5.85); mean time from diagnosis to follow- up MHOS was 12.4 months; treatments not discussed | SF-36 | NHL svrs (n = 53) had the greatest decline in physical health compared to other cancers; NHL svrs had lowered vitality and decrease in social function | Strengths: large, focused older adult sample, pt survey data linked with SEER data, evaluated HRQOL before and after cancer diagnosis. Limitations: Medicare managed-plan pts, sample size was small for NHL svrs, unable to conduct HRQOL follow-up survey (deaths, unenrolled from plan), no conceptual framework |
| Smith et al. (2010) [26] | Cross-sectional, NC Cancer Registry | Examine association between IOCv2 scales and outcomes in a large sample of adult NHL svrs | N = 652 NHL svrs, median age at study enrollment 62.7 years, mean age at diagnosis 51.9 years (SD 14.2), mean time from diagnosis to study enrollment 10.8 years (SD 7.5); RT: 48%, CT: 82% | IOC, SF-36, FACT-G | Svrs who were non- Caucasian, without a college degree, unemployed, younger at study enrollment, had comorbid conditions or less social support had worse QOL when controlled for other variables; comorbidities and negative appraisal reported worse physical and mental health (p < 0.05) | Strengths: validation of IOCv2; health status, functioning, and QOL in large sample of NHL svrs, broad demographic profile, high response rate (74%). Limitations: cross-sectional, two NC Comprehensive Cancer Centers, no conceptual framework |
| Smith et al. (2008) [24] | Cross-sectional, NC Cancer Registry | Estimate prevalence of PTSD symptoms in svrs. of adult NHL who are at least 2 years post- diagnosis and identify risk factors associated with PTSD symptoms | N = 886 NHL svrs, median age 52.6 years (range 25– 92), 2–44 years post- diagnosis; no treatment: 5%, surgery: 28%, RT: 47%, CT: 78%, BMT/SCT: 14.9%, biologic therapy: 29.5% | PCL-C, Medical Outcomes Study-Social Support Survey | 8% of svrs met PTSD diagnostic criteria and 39% had PTSD symptoms; the impact of a cancer diagnosis and treatment persists for years for many svrs; positive association between social support and QOL, p < 0.001 | Strengths: examined PTSD well-being of long-term NHL svrs, large sample with age range of 25–92, use of Lazarus & Folkman (1984) PTSD conceptual framework. Limitations: no comparison group, cross-sectional, svrs treated at two large, comprehensive cancer centers in southeastern region |
| Smith et al. (2009) [27] | Cross-sectional, NC Cancer Registry | Compare QOL status of those with active NHL to those who are disease- free, both short term (2– 4 years) and long term (≥5 years) post- diagnosis | N = 761 NHL svrs, median age 62.7 years (range 25– 92), 2–44 years post- diagnosis; surgery: 30.5%, RT: 47.8%, CT: 81.1%, BMT/SCT: 15.6%, biologic therapy: 28.3% | SF-36, FACT-Lym, IOC | Svrs with active disease had worse physical and mental health functioning, worse QOL, and less positive and more negative IOCs compared with disease- free svrs, p < 0.001 | Strengths: large sample, large older adult population, use of Lazarus & Folkman (1984) and cancer survivorship based on coping theories conceptual framework. Limitations: cross- sectional, two large comprehensive cancer centers |
| Vallance et al. (2005) [29] | Cross-sectional; retrospective survey, Canada | (1) Examine differences in QOL between NHL svrs who meet/do not meet exercise guidelines; (2) examine exercise behavior changes across treatment | N = 438 NHL svrs, mean age 61.1 years (SD 13.1); CT: 64.6%, RT: 10.7%, CT + RT: 15.5%, surgery: 3.6%, immunotherapy: 0.9%, BMT: 25.2%, watchful waiting: 17.1% | FACT-An | NHL svrs (n = 23) who met the public health exercise guidelines had better physical functioning (less fatigue), fewer anemia related symptoms, better mental functioning than those that did not meet the guidelines (n = 332) | Strengths: large population base of NHL svrs, use of well-established measures. Limitations: selection bias, recall bias related to length of long- term survivorship, self- report of exercise, observational study, no conceptual framework |
| Zebrack (2000) [39] | Cross-sectional, face-to-face semistructured interviews, California | Examine QOL of leukemia and lymphoma long-term svrs | N =53 long-term svrs, n =25 lymphoma svrs, median age at diagnosis 17, average years post-diagnosis 18, treatments not discussed | QOL-CS | Lymphoma svrs reported positive experiences from their cancer, uncertainties about the future were associated with decreased QOL, spiritual/existential QOL domain reflected that having a purpose in life is critical in survival | Strengths: qualitative description of quality of life, mixed methods approach. Limitations: cross-sectional, no comparison group, selection bias, no conceptual framework |
| Zebrack et al. (2008) [25] | Cross-sectional, California | Examine breast, prostate, colorectal, and lymphoma impact on QOL of long-term svrs | N =193 cancer svrs, n =49 lymphoma svrs, (Hodgkin and NHL), mean age at study 61.5 years (SD 14.3), 5–10 years post-diagnosis; treatments not discussed | SF-36, IOC, QOL-CS | Older svrs reported better overall QOL (p =0.004) and mental health (p < 0.001), but worse physical health (p =0.04); svrs reporting low income (p =0.02) and comorbidities (p =0.003) indicated worse physical functioning; higher negative IOC score was associated with worse physical functioning (p < 0.0001), worse mental health (p < 0.0001), and lower overall QOL (p < 0.0001); higher positive IOC score was associated with better mental health (p =0.0004) and better overall QOL (p =0.005) | Strengths: IOC instrument as potential tool, long-term svrs of breast, prostate, colorectal, and lymphoma. Limitations: cross-sectional, younger age at enrollment, younger age at diagnosis, high levels of education and income, sociodemographic variables were limited; no conceptual framework |
BMT/SCT, bone marrow transplant/stem cell transplant; CARES, Cancer Rehabilitation Evaluation System; CT, chemotherapy; EORTC, European Organisation for Research into the Treatment of Cancer; FACT-An, Functional Assessment of Cancer Therapies-Anemia; FACT-G, Functional Assessment of Cancer Therapies-General; FACT-Lym, Functional Assessment of Cancer Therapies-Lymphoma; FAHI, Functional Assessment of HIV Infection; HIV−, negative for human immunodeficiency virus; HIV+, positive for human immunodeficiency virus; HR, health-related; IOC, Impact of Cancer; IOCv2, Impact of Cancer scale version 2; lym., lymphoma; MHOS, Medical Health Outcomes Survey; NC, North Carolina; PCL-C, Post-Traumatic Stress Disorder Checklist-Civilian Version; PCP, primary care physician; pt, patient; PTSD, post-traumatic stress disorder; QLQ C-30, Quality of Life Questionnaire Cancer-30; QOL, quality of life; QOL-CS, Quality of Life-Cancer Survivors’ Tool; RT, radiation therapy; SD, standard deviation; SEER, Surveillance and Epidemiology End Results; SF-36, Short Form-36; svrs, survivors.
Physical well-being
Multiple chronic conditions can affect functional abilities and can decrease a person’s ability to maintain a healthy lifestyle. Using the Impact of Cancer (IOC) scale, Zebrack et al. [25] measured QOL in a sample of individuals with cancer (including 49 lymphoma survivors) who were 5–10 years post-diagnosis (n =193). They found that older adults reported better overall QOL (p =0.004) but worse physical health (p =0.04) than their younger counterparts. Lower income (p =0.02) and comorbidities (p =0.003) were associated with worse physical functioning. A higher negative impact summary score was associated with worse physical functioning (p <0.0001), worse mental health (p <0.0001), and lower overall QOL (p <0.0001).
These findings were consistent with the results of Smith et al. [26]. They measured NHL survivors’ (n =652) perceptions of positive and negative impact of cancer domains related to health status and QOL and found that survivors who were non-Caucasian, without a college degree, unemployed, younger at study enrollment, or had comorbid conditions or less social support also had lower QOL. In a related study, survivors with active NHL disease were found to have worse physical functioning and QOL than disease-free survivors [27].
While cancer-related detriments to physical functioning are common, it has been shown that healthy behaviors such as physical activity can improve QOL and reduce symptoms such as fatigue in cancer survivors [28]. In a cross-sectional study of NHL survivors (n =438), differences in overall QOL were found between NHL survivors who met and did not meet recommended public health exercise guidelines of at least 150 min per week of moderate to strenuous activity [29]. The NHL survivors who met these guidelines reported higher QOL than those who did not meet them, suggesting that individuals who exercise at least 150 min per week receive physical and mental health benefits [30]. Bellizzi et al. [31] also explored physical activity as it related to QOL and found that survivors who engaged in some form of physical activity reported better physical and mental health (p <0.001). Furthermore, Reeve et al. [32] examined QOL among older adults using the Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey in a longitudinal, population-based study. Changes in QOL from before and after the cancer diagnoses were reported in nine cancer groups including NHL; the NHL survivors (n =53) had one of the highest declines in physical health. In summary, these studies highlight that older adult cancer survivors and those with comorbid illnesses experience more physical health problems and worse physical QOL compared to their younger counterparts and healthy controls; however, physical activity can provide some protective effects and improve QOL in these individuals.
Psychological well-being
Psychological changes occur during the survivorship trajectory and can be stressful on the mind and body, resulting in negative health outcomes for the survivor. How an individual appraises his or her diagnosis or the perceived threat of the cancer can affect clinical and psychological outcomes [33]. The Diagnostic and statistical manual of mental disorders, 4th edition, recognizes that a cancer diagnosis meets the criteria of a traumatic stressor [34]. For example, survivors of NHL may develop symptoms of posttraumatic stress disorder (PTSD) that are also reported in individuals who experience a non-cancer-related traumatic event (e.g. motor vehicle accident, sexual assault, military combat) [23,24].
In a cross-sectional study of NHL survivors (n =886), 8% met the criteria for a full PTSD diagnosis (compared to an estimated 2.4% prevalence in the general adult population), 9.1% met partial criteria, and 39% of the total sample had PTSD symptoms in at least one of the three domains used for diagnosis [24]. In addition, Geffen et al. [23] found that NHL survivors who had more PTSD symptoms also experienced lower physical QOL.
In other studies, lymphoma survivors were found to have mental health functioning comparable to the general population [31]. However, survivors with active NHL disease had worse mental health functioning than those individuals in remission. In addition, younger NHL survivors (25–34 and 45–55 years of age) had lower mental summary scores than the general population [27]. In summary, it has been shown that a cancer diagnosis affects an individual’s psychological well-being and their ability to cope during a time of uncertainty.
Social well-being
Social well-being encompasses the interpersonal relationships that may have been changed by the cancer experience as the survivor reintegrates into the social environment. Aside from receiving the diagnosis, the treatment for NHL can have a negative effect on various QOL domains, including changes in social relationships. Social functioning after a cancer diagnosis may be a long-term adjustment for survivors, warranting further exploration.
In one study, Bellizzi et al. [31] reported that NHL had a positive effect on relationships but a negative effect on a survivor’s current financial situation. The social impact of financial and relationship changes led survivors to other sources of social support including formal and informal sources. Social support was a significant positive psychosocial variable with a mean of 83.1 (SD 16.4) on a 20–100 scale. This number indicates that many NHL survivors perceive to have supportive resources or individuals that are important to them during times of unpredictability and change [27]. Employment, financial, and insurance issues are also ongoing concerns of survivors and are addressed in the ‘Functional well-being’ section below.
Functional well-being
The Functional Assessment of Cancer Therapy-General (FACT-G) measure includes functional well-being as one of its QOL domains. Six of the studies used the FACT-G instrument with consistent findings that one or more health problems coupled with NHL can lead to a decline in functional well-being. Chronic conditions can impede a survivors’ ability to maintain healthy behaviors and lifestyles, further contributing to a decline in function. Hewitt et al. [13] reported that cancer survivors, including those of NHL, have an almost two-fold likelier chance of having at least one functional limitation. When an additional chronic condition is combined with cancer, functional limitation increases. In addition, Diamond et al. [20] reported that HIV-infected survivors with NHL have worse overall QOL and survival rates than uninfected survivors with NHL, indicating that having more than one life-limiting illness had a detrimental effect on the overall health and survival of their study participants.
Smith et al. [27] reported that 60% of NHL survivors were either unemployed or retired due to issues related to their cancer. In addition, how NHL survivors perceive their illness can influence their functional and physical abilities, which can impede their QOL [26]. Functional change at any juncture of the survivorship journey may require a change in living arrangements and the amount of support needed. This QOL domain continues to gain more interest as the population ages, in terms of the related impacts on daily living.
In one study, survivors who were able to maintain their work status reported higher physical QOL compared to those who reduced their work hours or stopped working completely [35]. Forty-one percent of the participants in the same study reported a change in work status and related QOL decline due to their cancer, along with problems obtaining insurance or a mortgage.
Not only do NHL survivors experience physiological effects with risks of disability and poor functioning, but also psychological stresses of healthcare expenditures on their QOL. In addition, individuals with chronic health conditions (including cancer survivors) account for the majority of US healthcare expenses. Assessment of QOL may direct interventions that reduce healthcare costs and, ultimately, improve QOL [36].
Spiritual and existential well-being
Cancer survivors’ experiences parallel those of individuals who have experienced other traumatic stressors, involving acute, unpredictable events that threaten an individual’s life. However, few studies have assessed the positive psychological changes or benefits from the illness [31,37]. In one of these studies, the majority of cancer survivors reported that experiencing a traumatic event, such as the diagnosis of a life-threatening illness, led to positive personal changes, a greater ability to cope with life stressors, and a sense of personal strength [33]. Posttraumatic growth is an interrelated concept that has been linked with the spiritual and existential QOL domain, or a ‘perceived positive psychological change experienced as a result of the struggle with highly challenging life circumstances’ [38]. Exploration of the role of spirituality has increased in recent years, but is typically not addressed or described in research studies.
In addition, having cancer often prompts individuals to revisit their outlook on life. Zebrack’s study [25] used a qualitative approach with face-to-face, semistructured interviews to explore four QOL domains (physical, psychological, social, and spiritual or existential). The spiritual and existential domain of QOL was reflected in finding purpose and meaning in life, both of which were critical components in the survivorship trajectory. Zebrack et al. [25] found that having a purpose in life and hopefulness were highly rated spiritual subscale items, leading to a conclusion that survivors who are hopeful have a higher QOL.
Treatment effects on QOL
Watch-and-wait or active surveillance approaches for indolent types of NHL are the most conservative management strategies. Surveillance involves monitoring through blood tests and physical examinations until the symptoms progress or the cancer interferes with the survivor’s QOL [39]. Individuals with fast-growing or aggressive NHL often present with symptoms such as fatigue, weight loss, night sweats, or complaint of sore lymph nodes [40]. Aggressive NHL is commonly treated with targeted biologic therapies such as rituximab.
Most NHL survivors included in these studies received chemotherapy, radiation therapy, rituximab, or some combination of them for their treatment. The NHL survivors treated with chemotherapy or with active disease reported significantly worse psychological and social well-being and health-related QOL than those who had not received chemotherapy [20,38]. The survivors who reported significantly less vitality and worse general health compared with the general population [32,41], and those treated with either radiation therapy or watchful waiting, did not report a worse QOL compared with the general population [42]. Survivors who relapsed had worse QOL and physical and mental functioning compared to newly diagnosed NHL survivors [43].
Persson and Hallberg [44] explored the experience of receiving lymphoma and leukemia treatment using a phenomenological design. Three themes emerged from their research about what survivors felt while undergoing treatment: belief in life (they fought for it and came through stronger); life went on (they adapted and found a balance in the new life); or life was over (they felt out of control and lost belief in life). The impact of the disease varied depending on the survivor’s illness trajectory and their ability to cope with the disease’s unpredictability. This study provided meaning of the experiences felt by lymphoma and leukemia survivors and allowed them to retell their story of living with the disease.
Older adults with NHL
Age-related issues that impact QOL for NHL survivors were examined in fewer than 10 studies. For those that did, older age was associated with worse physical QOL but better mental health compared with younger adults with NHL [16,27,32,39, 41,45,46]. Most of the survivors studied received chemotherapy in combination with radiation therapy, surgery, bone marrow transplant, stem cell transplant, or biological therapies. Additionally, older survivors who received chemotherapy had poorer psychological and social well-being compared to those who did not receive chemotherapy [16,35].
It is not uncommon that older adults have one or more coexisting illnesses coupled with cancer [15], which is a finding that needs to be explored. Further research within this population will provide new insights into how older NHL survivors manage their multiple comorbidities and the impact on their QOL.
Design and framework
The design and framework of a study impacts the quality of the data and how it relates to other areas of research. The methods used in the reviewed studies (Table II) had both negative and positive effects on the ability to use the data as an accurate measurement of the QOL in NHL survivors. Large, cross-sectional studies are generally less costly than longitudinal studies but are limited to describing only associations within the sample. For example, cross-sectional designs do not answer cause and effect but provide useful information in the exploration of relationships among QOL domains and the effects of cancer treatments.
Table II.
QOL-related measures used in NHL survivorship studies (n = 6).
| QOL measure | Number of items | Content or subscales | Reliability | Validity |
|---|---|---|---|---|
| SF-36 (Ware & Sherbourne, 1992) [52] | 36 | Generic measure of QOL | Test–retest, α > 0.70 | Observed physical outcomes correlate with scale |
| FACT-G (Cella et al., 1993) [18] | 33 | Cancer specific; physical, functional, social/familial, and emotional well-being | Test–retest, α > 0.70 | Discriminates on medical variables |
| FACT-Lym (Cella et al., 2005) [53] | 22 | Cancer specific; physical, functional, social/familial, and emotional well-being focused on NHL | Test–retest, α > 0.70 | Discriminates on medical variables |
| FACT-An (Cella, 1997) [54] | 20 | Cancer specific; physical, functional, emotional, and social well-being; anemia symptoms | Test–retest, α > 0.70 | Discriminates on medical variables |
| The City of Hope QOL-Cancer Survivors(Ferrell et al., 1995; Ferrell et al., 1995) [55,56] | 41 | Cancer specific; physical, psychological, social, and spiritual well-being | Test–retest, α > 0.70 | Discriminates on medical and demographic variables |
| Impact of Cancer (version 2) (Crespi et al., 2008) [57] | 50 | Cancer specific; Positive Impact Scale (altruism and empathy, health awareness meaning of cancer, positive self-evaluation); Negative Impact Scale (appearance concerns, body change concerns, life interferences, worry); employment concerns; relationship concerns (partnered); relationship concerns (not partnered) | Test–retest, α > 0.70 | Discriminates on medical and demographic variables |
FACT-An, Functional Assessment of Cancer Therapy-Anemia; FACT-G, Functional Assessment of Cancer Therapy-General; FACT-Lym, Functional Assessment of Cancer Therapy-Lymphoma; NHL, non-Hodgkin lymphoma; QOL, quality of life; SF-36, Short Form-36.
The lack of a conceptual or theoretical model can be problematic when QOL is used to guide the study’s design or is viewed as the outcome variable. While theoretically driven studies allow relationships to be tested in the model, only three reviewed studies identified conceptual or theoretical perspectives [24,27,45]. Inclusion of a model addressing the multidimensionality of QOL and its interrelationships or factors among QOL domains allows for hypotheses and relationships to be tested [47]. Using theoretically based models of QOL increases applicability of the concept and contributes to the ongoing development of cancer survivorship models. Ultimately, intervention studies to improve QOL in NHL survivors could be developed based on these models.
Discussion
This article provides an integrative review of the survivorship literature relevant to older NHL survivors with a focus on the QOL measures and treatment methods used. While a limited number of studies examining QOL factors of older NHL survivors were found, the literature reflected growing interest in understanding the QOL among NHL survivors as a group. This is evidenced by recently published research (within the last decade) from around the world. Limitations of this literature review include underrepresentation of minority NHL survivors, lack of longitudinal studies and theoretical or conceptual frameworks, limited findings specific to older adults, and lack of literature regarding long-term survivors. In other cancer groups, racial and/or ethnic differences in QOL have been reported; therefore, future NHL studies with increased minority representation may find similar disparities [48]. Also, qualitative studies are needed to enhance the understanding of the impact that NHL has on QOL for survivors, particularly in minority and older populations. Finally, mixed methods studies that complement quantitative findings and explore the experiences of NHL survivors, such as Zebrack’s [25] study, in which survivors shared their experiences of receiving their cancer diagnoses and their uncertainties about the future associated with decreased QOL, would be beneficial. This is one example of how quantitative and qualitative methods informed one another, at least in the spiritual and existential domain of QOL.
While QOL was measured objectively in all of the studies, it is also important to understand the subjective nature of the multidimensionality of QOL, which allows for survivors to express their experiences more thoroughly. Using qualitative methods to complement standardized QOL-related instruments would more adequately capture the totality of a survivor’s experience compared with using one measure.
Implication for future research
Cancer survivors are at increased risk for QOL-related concerns due to their exposure to disease and treatment-related effects (e.g. recurrences, secondary malignancies, cardiotoxicities, social and financial issues) compared to the general population [10]. Lack of understanding of the QOL outcomes (physical, psychological, social, functional, and spiritual/existential) of older adults with cancer is a growing health concern due to the aging of the US population. For survivors of all ages, there is increasing information on intermediate (2–5 years post-diagnosis) and longer-term (>5 years post-diagnosis) adjustment to NHL. Increasing sociodemographic diversity in the sample could also enhance the generalizability of the findings.
As the growing population of older NHL survivors increases, research focused on QOL-related outcomes is needed. Future studies are needed to build on descriptive, cross-sectional designs and provide a more comprehensive understanding of the survivorship journey. Longitudinal designs in cancer survivorship can help specify under what circumstances the process of cancer survivorship adaptation is best described [6]. From these longitudinal findings, interventions tailored specifically to NHL survivors will determine who responds to the intervention and the sustainable effect of the intervention. Study findings could be generalized also to a larger, long-term, survivor population.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.American Cancer Society. Cancer facts & figures [Internet] 2010 Available from: http://www.cancer.org/downloads/STT/Cancer_Fact_and_Figures_2010.pdf.
- 2.Horner M, Ries L, Krapcho M, editors. SEER Cancer Statistics Review, 1975–2006 [Internet] National Cancer Institute; Available from: http://seer.cancer.gov/csr/1975_2006. [Google Scholar]
- 3.Cote T, Biggar R, Rosenberg P, et al. Non-Hodgkin’s lymphoma among people with AIIDS: incidence, presentation and public health burden. Int J Cancer. 1997;73:645–650. doi: 10.1002/(sici)1097-0215(19971127)73:5<645::aid-ijc6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Non-Hodgkin lymphoma [Internet] 2010 Available from: http://www.cancer.gov/cancertopics/types/non-hodgkin.
- 5.Hamblin T. Achieving optimal outcomes in chronic lymphocytic leukaemia. Drugs. 2001;61:593–611. doi: 10.2165/00003495-200161050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Aziz N, Rowland J. Trends and advances in cancer survivorship research: challenge and opportunity. Semin Radiat Oncol. 2003;13:248–266. doi: 10.1016/S1053-4296(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 7.Bloom J. Surviving and thriving? Psychooncology. 2002;11:89–92. doi: 10.1002/pon.606. [DOI] [PubMed] [Google Scholar]
- 8.Brown J, Byers T, Doyle C, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2003;53:268–291. doi: 10.3322/canjclin.53.5.268. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Cancer survivorship—United States, 1971–2001. MMWR Morb Mortal Weekly Rep. 2004;53:526–529. [PubMed] [Google Scholar]
- 10.Ganz P. Late effects of cancer and its treatment. Semin Oncol Nurs. 2001;17:241–248. doi: 10.1053/sonu.2001.27914. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Clegg L, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 12.Bolin K. Health among long-term survivors of breast cancer: an analysis of 5-year survivors based on the Swedish surveys of living conditions 1979–1995 and the Swedish Cancer Registry 2000. Psychooncology. 2008;17:1–8. doi: 10.1002/pon.1189. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt M, Rowland J, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 14.Yancik R, Ganz P, Varricchio C, et al. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. J Clin Oncol. 2001;19:1147–1151. doi: 10.1200/JCO.2001.19.4.1147. [DOI] [PubMed] [Google Scholar]
- 15.Yancik R, Havlik R, Wesley M, et al. Cancer and comorbidity in older patients: a descriptive profile. Ann Epidemiol. 1996;6:399–412. doi: 10.1016/s1047-2797(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 16.Mols F, Coebergh J, van de Poll-Franse L. Health-related quality of life and health care utilisation among older long-term cancer survivors: a population-based study. Eur J Cancer. 2007;43:2211–2221. doi: 10.1016/j.ejca.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. Non Hodgkin’s lymphoma overview [Internet] 2010 Available from: http://www.nccn.com/treatment-summaries/non-hodgkins-lymphoma.html.
- 18.Cella D, Tulsky D, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly S. Quality-of-life assessment in advanced cancer. Curr Oncol Rep. 2000;2:338–342. doi: 10.1007/s11912-000-0027-7. [DOI] [PubMed] [Google Scholar]
- 20.Diamond C, Taylor T, Anton-Culver H. Quality of life, characteristics and survival of patients with HIV and lymphoma. Qual Life Res. 2010;19:149–155. doi: 10.1007/s11136-009-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherbourne C, Stewart A. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 22.Bellizzi K, Miller M, Arora N, et al. Positive and negative life changes experienced by survivors of non-Hodgkin’s lymphoma. Ann Behav Med. 2007;34:188–199. doi: 10.1007/BF02872673. [DOI] [PubMed] [Google Scholar]
- 23.Geffen D, Blaustein A, Amir M, et al. Post-traumatic stress disorder and quality of life in long-term survivors of Hodgkin’s disease and non-Hodgkin’s lymphoma in Israel. Leuk Lymphoma. 2003;44:1925–1929. doi: 10.1080/1042819031000123573. [DOI] [PubMed] [Google Scholar]
- 24.Smith S, Zimmerman S, Williams C, et al. Post-traumatic stress outcomes in non-Hodgkin’s lymphoma survivors. J Clin Oncol. 2008;26:934–941. doi: 10.1200/JCO.2007.12.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zebrack B, Yi J, Petersen L, et al. The impact of cancer and quality of life for long-term survivors. Psychooncology. 2008;17:891–900. doi: 10.1002/pon.1300. [DOI] [PubMed] [Google Scholar]
- 26.Smith S, Crespi C, Petersen L, et al. The impact of cancer and quality of life for post-treatment non-Hodgkin lymphoma survivors. Psychooncology. 2010;19:1259–1267. doi: 10.1002/pon.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith S, Zimmerman S, Williams C, et al. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer. 2009;115:3312–3323. doi: 10.1002/cncr.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto B, Rabin C, Abdow S, et al. A pilot study on disseminating physical activity promotion among cancer survivors: a brief report. Psychooncology. 2008;17:517–521. doi: 10.1002/pon.1268. [DOI] [PubMed] [Google Scholar]
- 29.Vallance J, Courneya K, Jones L, et al. Differences in quality of life between non-Hodgkin’s lymphoma survivors meeting and not meeting public health exercise guidelines. Psychooncology. 2005;14:979–991. doi: 10.1002/pon.910. [DOI] [PubMed] [Google Scholar]
- 30.Pate R, Pratt M, Blair S, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 31.Bellizzi K, Rowland J, Arora N, et al. Physical activity and quality of life in adult survivors of non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:960–966. doi: 10.1200/JCO.2008.17.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeve B, Potosky A, Smith A, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tedeschi R, Park C, Calhoun L. Posttraumatic growth: conceptual issues. In: Tedeschi R, Park C, Calhoun L, editors. Posttraumatic growth: positive changes in the aftermath of crisis. Mahwah, NJ: Erlbaum; 1998. pp. 1–22. [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA; 2000. [Google Scholar]
- 35.Mols F, Thong M, Vreugdenhil G, et al. Long-term cancer survivors experience work changes after diagnosis: results of a population-based study. Psychooncology. 2009;18:1252–1260. doi: 10.1002/pon.1522. [DOI] [PubMed] [Google Scholar]
- 36.Baker F, Haffer S, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97:674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 37.Tomich P, Helgeson V. Is finding something good in the bad always good? Benefit finding among women with breast cancer. Health Psychol. 2004;23:16–23. doi: 10.1037/0278-6133.23.1.16. [DOI] [PubMed] [Google Scholar]
- 38.Stanton A, Bower J, Low C. Posttraumatic growth after cancer. In: Calhoun L, Tedeschi R, editors. Handbook of posttraumatic growth: research and practice. Mahwah, NJ: Erlbaum; 2006. pp. 138–175. [Google Scholar]
- 39.Zebrack B. Cancer survivors and quality of life: a critical review of the literature. Oncol Nurs Forum. 2000;27:1395–1401. [PubMed] [Google Scholar]
- 40.Elphee E. Understanding the concept of uncertainty in survivors with indolent lymphoma. Oncol Nurs Forum. 2008;35:449–454. doi: 10.1188/08.ONF.449-454. [DOI] [PubMed] [Google Scholar]
- 41.Mols F, Aaronson N, Vingerhoets A, et al. Quality of life among long-term non-Hodgkin lymphoma survivors. Cancer. 2007;109:1659–1667. doi: 10.1002/cncr.22581. [DOI] [PubMed] [Google Scholar]
- 42.Bailey D, Mishel M, Belyea M. Uncertainty intervention for watchful waiting in prostate cancer. Cancer Nurs. 2004;27:339–346. doi: 10.1097/00002820-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Pettengell R, Donatti C, Hoskin P. The impact of follicular lymphoma on health-related quality of life. Ann Oncol. 2008;19:570–576. doi: 10.1093/annonc/mdm543. [DOI] [PubMed] [Google Scholar]
- 44.Persson L, Hallberg I. Lived experience of survivors of leukemia or malignant lymphoma. Cancer Nurs. 2004;27:303–313. doi: 10.1097/00002820-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Arora N, Hamilton A, Potosky A, et al. Population-based survivorship research using cancer registries: a study of non-Hodgkin’s lymphoma survivors. J Cancer Surviv. 2007;1:49–63. doi: 10.1007/s11764-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 46.Kouroukis T, Meyer R, Benger A, et al. An evaluation of age-related differences in quality of life preferences in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2004;45:2471–2476. doi: 10.1080/10428190400002285. [DOI] [PubMed] [Google Scholar]
- 47.Aaronson N, Meyerowitz B, Bard M. Quality of life research in oncology. Past achievements and future priorities. Cancer. 1991;67:839–843. doi: 10.1002/1097-0142(19910201)67:3+<839::aid-cncr2820671415>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Chlebowski R, Chen Z, Anderson G, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 49.Ahles T, Saykin A, Furstenberg C, et al. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J Clin Oncol. 2005;23:4399–4405. doi: 10.1200/JCO.2005.03.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arden-Close E, Pacey A, Eiser C. Health related quality of life in survivors of lymphoma: a systematic review and methodological critique. Leuk Lymphoma. 2010;51:628–640. doi: 10.3109/10428191003587263. [DOI] [PubMed] [Google Scholar]
- 51.Crespi C, Smith S, Petersen L, et al. Measuring the impact of cancer: a comparison of non-Hodgkin lymphoma and breast cancer survivors. J Cancer Surviv. 2010;4:45–58. doi: 10.1007/s11764-009-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ware J, Sherbourne C. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 53.Cella D, Webster K, Cashy J, et al. Development of a measure of health-related quality of life for non-Hodgkin’s lymphoma clinical research: the Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) Blood. 2005;106(Suppl 1):Abstract 750. [Google Scholar]
- 54.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(Suppl 2):13–19. [PubMed] [Google Scholar]
- 55.Ferrell B, Dow K, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum. 1995;22:915–922. [PubMed] [Google Scholar]
- 56.Ferrell B, Dow K, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 57.Crespi C, Ganz P, Petersen L, Castillo A, Caan B. Refinement and psychometric evaluation of the Impact of Cancer Scale. J Natl Cancer Inst. 2008;100:1530–1541. doi: 10.1093/jnci/djn340. [DOI] [PMC free article] [PubMed] [Google Scholar]


