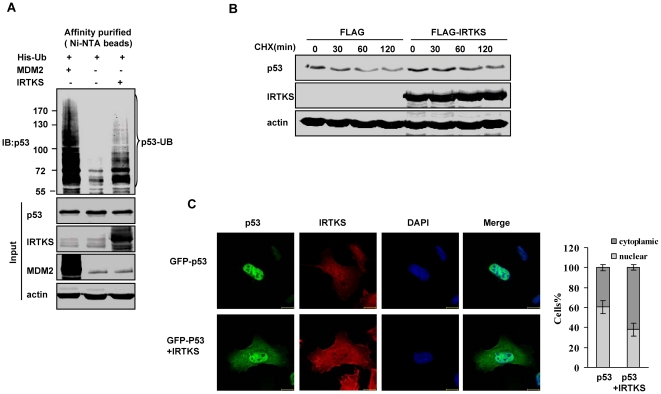
Figure 3. IRTKS enhanced the ubiquitination and cytoplasmic localization of p53.
(A) In vivo ubiquitination assay revealed IRTKS-induced p53 ubiquitination. U2OS cells were co-transfected with a 6XHis-Ub expression vector and IRTKS (lane 3). The cell lysates were loaded on nickel (Ni+)-NTA columns. p53 ubiquitination was analyzed with Western blotting by using anti-p53 antibody (DO-1). MDM2 was used as a positive control for p53 ubiquitination (lane1). Note that the IRTKS-induced p53 ubiquitination was in lower molecular weights (<130 kD). (B) IRTKS overexpression resulted in a moderate stabilization of endogenous p53 protein. Half-life of p53 protein was measured in cycloheximide (CHX) treated U2OS cells transfected with FLAG-IRTKS and control plasmid. Protein extracts, prepared at indicated time points after CHX addition, were analyzed by Western blotting. (C) IRTKS increased the cytoplasmic localization of p53. U2OS cells transfected with GFP-p53 and FLAG-IRTKS were stained for IRTKS. The nuclei were counterstained with DAPI and images were taken through a confocal microscope. One hundred cells were counted for each treatment in two separate experiments. The cells with higher levels of GFP-p53 in the cytoplasm versus the nucleus (dark gray bars) and higher levels of p53 in the nucleus versus the cytoplasm (gray bars) were scored.