Fig. 2.
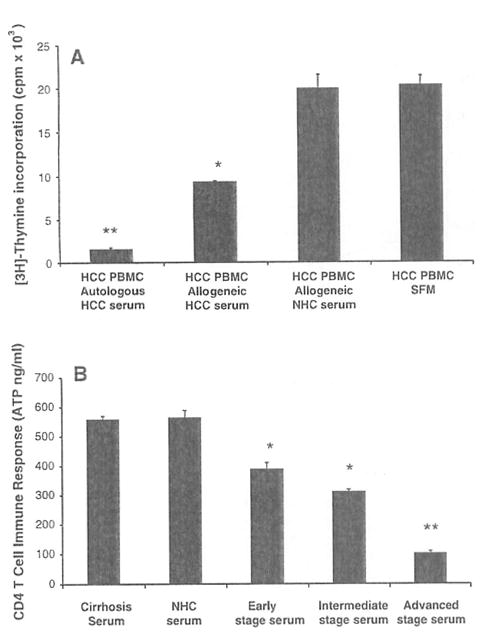
Hepatocellular carcinoma (HCC) serum suppresses effector T cell responses, a PBMCs from patients with advanced stage C HCC (n = 5) were cultured with autologous HCC serum, allogeneic HCC serum, allogeneic healthy subject (NHC) serum and in serum free medium (SFM) with PHA to measure effector proliferation by [3H] thymidine incorporation. Results are shown as mean ± standard error (SE) counts per minute (cpm × 103). The cultures with autologous and allogeneic HCC serum showed significantly lower proliferation when compared to the cultures with allogeneic NHC serum and SFM. b CD4 T cell immune responses from NHCs (n = 5) were measured ex vivo after culture with a constant aliquot of HCC serum from three stages of HCC (early, intermediate, and advanced) with the ImmuKnow™ ATP based assay. Results are shown as mean CD4 T cell immune response ± SE (ATP ng/ml). In general, the CD4 T cell reactivity by NHCs decreased in a graded fashion with worsening stage of HCC with maximal suppressive effect seen with the advanced HCC serum and the least suppressive effect seen with the early HCC serum. The suppressive effect of HCC serum appears to be HCC specific since it was not observed with serum from patients with HCV related cirrhosis (559.5 ± 9.51; n = 5). * P < 0.05, ** P < 0.01
