Fig. 3.
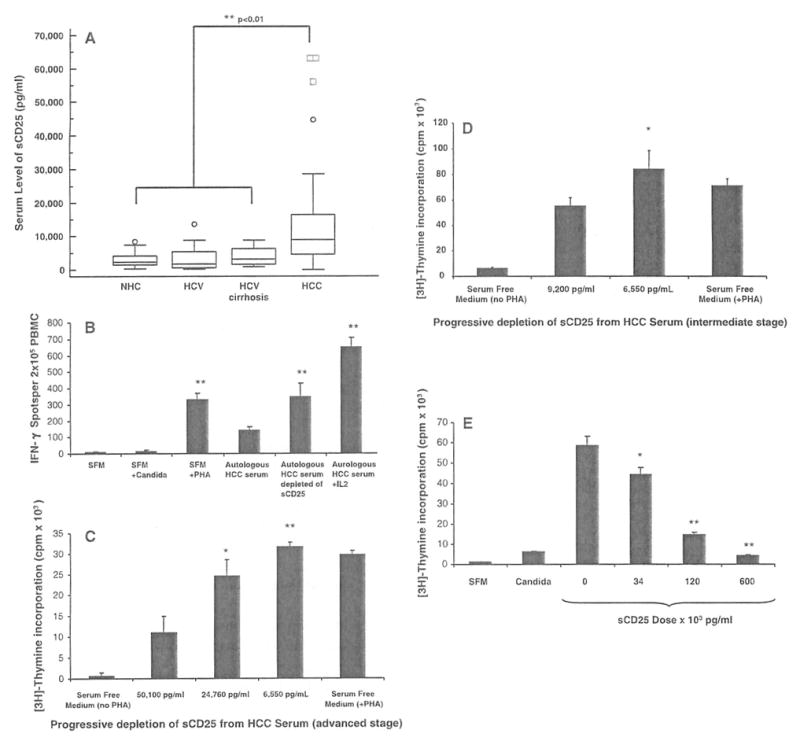
Serum levels of sCD25 and effect of sCD25 depletion and supplementation on T cell responses, a Levels of sCD25 in the serum of healthy subjects (NHC; n = 30), chronic HCV infection (HCV; n = 40), HCV-related cirrhosis (n = 20) and hepatocellular carcinoma (HCC) patients (n = 60) were determined using an ELISA assay. Data are expressed as box plots of the serum levels of sCD25 (pg/ml) for the four study groups. The average levels of sCD25 are significantly higher in HCC subjects (mean ± SE; 20,701 ± 4,018 pg/ml, P < 0.01) when compared to NHC (2,953 ± 378 pg/ml, P < 0.01) and disease controls (HCV 4,604 ± 608 pg/ml, P < 0.01; HCV cirrhosis 3,775.6 ± 534 pg/ml, P < 0.01). b Depletion of sCD25 enhances IFN-γ ELISpot responses. IFN-γ ELISpot responses were assessed using PBMC from patients with advanced HCC (n = 5) cultured with autologous serum with and without IL-2 (500 units/ml), autologous serum depleted of sCD25, and in serum free medium (SFM) with (PHA, Candida) and without stimulation. Results are expressed as means ± SE IFN-γ spots per 2 × 105 PBMCs. Depletion of sCD25 from the serum was performed with the use of the anti-sCD25 plated ELISA assay. The mean IFN-γ response obtained from the HCC PBMCs with autologous HCC serum prior to depletion (139 ± 24) was enhanced after depleting sCD25 from autologous HCC serum (348 ± 82, P < 0.01), when cultured with SFM plus PHA (329 ± 40, P < 0.01) and when high doses of IL 2 (500 units/ml) were added to the autologous HCC serum cultures (650 ± 57, P < 0.01). This analysis shows that the suppressive effects of HCC serum on IFN-γ T cell secretion seen with HCC PBMCs appears reversible when the content of sCD25 in the HCC serum was reduced and by exogenous IL-2. c Depletion of sCD25 enhances CD4 T cell proliferation. Mean proliferative responses (cpm × 103) of CD4+ CD25− T cells from normal healthy subjects (NHC, n = 5) when cultured with SFM, with HCC serum before sCD25 depletion with a sCD25 concentration of 50,100 pg/ml, and with HCC serum after two sequential depletions of sCD25 yielding two concentrations (24,760 and 6,550 pg/ml). Progressive sCD25 depletion from HCC serum enhanced the proliferative responses of NHC CD4+CD25− T cells (11,422 vs. 24,536 vs. 31,622 cpm). The degree of suppression showed a dose-dependent pattern that appeared related to the levels of sCD25 in the HCC serum, d Depletion of sCD25 from the serum in patients with intermediate stage HCC (n = 5) similarly enhances proliferation of CD4+CD25− T cells from NHC from 55,577 ± 6,295 cpm (before sCD25 depletion with a sCD25 concentration of 9,200 pg/ml) to 84,595 ± 13,922 (after sCD25 depletion with a sCD25 concentration of 113 pg/ml). e Supplementation of recombinant sCD25 suppresses CD4 T cell proliferation. Mean proliferation of CD4+CD25− T cells from NHCs (n = 5) when stimulated with PHA in serum free medium (SFM) with no sCD25 (0 pg/ml) and with increasing doses of sCD25 (34, 120 and 600 × 103 pg/ml) to characterize its effect on T cell proliferation by [3H] thymidine incorporation (mean ± SE cpm × 103). Recombinant sCD25 suppresses the mean proliferative response of CD4+CD25− T cells in a dose-dependent manner: 58,518 ± 4,709 cpm at baseline with no sCD25 (0 pg/ml) versus 44,109 ± 3,584 cpm (34 × 103 pg/ml) versus 14,667 ± 1,030 cpm (120 × 103 pg/ml) versus 4,331 ± 302 cpm (600 × 103 pg/ml). * P < 0.05, ** P < 0.01
