Abstract
Objective
Self-rated health is a strong independent predictor of mortality after accounting for objective health status, behavioral risk factors, and sociodemographic characteristics. However, mechanisms underlying this association are largely unexplained. Inflammation has been associated with increased risk of morbidity and mortality in the elderly. The current study aimed to: 1) examine associations between self-rated health and serum inflammatory markers in older adults; 2) examine the relative strength of these associations for self-rated health versus self-rated change in recent health; 3) examine components of self-rated health that may underlie the association between inflammation and global self-rated health.
Methods
Self-rated health, as measured by the RAND health survey, and serum interleukin (IL)-6 and C-reactive protein (CRP) were assessed among 250 generally healthy older adults (185 women, 65 men; average age= 63.8 ± 13.7 years).
Results
A series of linear regression analyses demonstrated that poorer self-rated health was significantly associated with higher IL-6 and CRP. These relationships remained after controlling for age, body mass index, gender, and objective health conditions. These associations also remained after controlling for depressive symptoms, neuroticism, perceived change in health over the past year, and health behaviors (smoking, sleep quality, and physical activity). Analyses of RAND component measures demonstrated that poorer physical functioning was significantly associated with IL-6; the relationship between global self-rated health and both IL-6 and CRP remained after accounting for perceived physical functioning.
Conclusions
Poorer self-rated health is associated with elevated serum inflammatory markers among generally healthy older adults. The relationship of self-rated health with inflammatory markers is not secondary to depressive symptoms, neuroticism, or recent changes in perceived health. Subjective ratings of health provide important clinical information regarding inflammatory status, beyond traditional objective risk factors, even among generally healthy individuals.
Keywords: self-rated health, perceived health, cytokines, interleukin-6, CRP, inflammation, sickness behavior, aging, psychoneuroimmunology
Self-rated health is a strong independent predictor of mortality after accounting for objective health status, behavioral risk factors, and sociodemographic characteristics. Single-item measures of self-rated health have predicted mortality among individuals with chronic diseases including diabetes (Dasbach et al. 1994) and cardiovascular disease (Bosworth et al. 1999) as well as dialysis patients (Thong et al. 2008) after controlling for objective indicators of disease severity. In addition, over two dozen studies across different countries have found associations between self-rated health and subsequent mortality in the general population (for review see Idler and Benyamini 1997; Benyamini and Idler 1999). Although differential effects based on gender, socioeconomic status, and race have been reported in some studies (Benyamini et al. 2003; Franks et al. 2003; Singh-Manoux et al. 2007; Yao and Robert 2008), overall the predictive value of self-rated health across diverse samples is surprisingly robust. Thus, single-item measures of self-rated health provide clinically relevant information regarding health status, above and beyond traditional objective indicators of health.
Mechanisms underlying the association between self-rated health and mortality are largely unexplained. A highly plausible pathway is inflammatory processes. Chronic elevations in serum proinflammatory cytokines contribute to the development of a number of serious health conditions including cardiovascular diseases, arthritis, diabetes, inflammatory bowel disease, periodontal disease, certain cancers, and age-related functional decline (Hamerman et al. 1999; Ershler and Keller 2000; Pedersen et al. 2000; Bruunsgaard et al. 2001; Black 2002; Ishihara and Hirano 2002). Moreover, elevated levels of circulating inflammatory markers predict increased risk of morbidity and mortality in the elderly (Harris et al. 1999; Bruunsgaard et al. 2003; Cappola et al. 2003; Roubenoff et al. 2003; Maggio et al. 2006). Notably, inflammation is well-known to induce symptoms of sickness behavior, including lethargy, decreased appetite, and behavioral withdrawal (Dantzer and Kelley 2007). Such symptoms may in turn affect subjective appraisals of health, even among generally healthy adults. In this manner, inflammation may be a common factor contributing to both reduced self-rated health and increased mortality risk.
Few studies have examined associations between self-rated health and inflammation. In a community sample of 1,727 elderly adults, a graded association between a single-item measure of self-rated health and IL-6 was reported; this association was much stronger than the association of IL-6 with any specific health diagnosis (Cohen et al. 1997). Among 265 primary health care patients, poorer self-rated health was associated with higher levels of inflammatory markers (IL-1β, IL-1ra, and TNF-α) among women, but not men (Lekander et al. 2004). Further, self-rated health was more strongly associated with inflammation than was physician-rated patient health. Subsequent research by the same group demonstrated that age moderated this relationship; among 174 primary health care patients, poorer self-rated health was associated with higher TNF-α among all age groups, while associations between self-rated health with IL-1β and IL-1ra were significant only among the oldest participants (Unden et al. 2007). Inflammatory markers (IL-6 and CRP) have also been associated with self-rated health among women with coronary heart disease after controlling for various health confounds (Janszky et al. 2005). Thus, available data indicate that self-rated health is associated with elevated serum proinflammatory proteins and that this relationship is more robust than and remains after controlling for objective indicators of health.
It has been argued that the predictive value of self-rated health for mortality may be a function of an individual’s perception of recent declines in health. However, evidence regarding the relative predictive value of self-rated health versus self-rated change in health in predicting mortality is limited and mixed. In a study of elderly adults, inclusion of perceived changes in health in the past 24 months weakened but did not fully account for the association between current self-rated health and mortality (Thomas et al. 1992). In contrast, among a sample of older adult men, when changes in self-rated health over two years were included in the model, current self-rated health was no longer a significant predictor of mortality (Wolinsky et al. 1993). To our knowledge, no studies have examined whether current perceptions of health versus perceptions of recent changes in health are more strongly associated with serum inflammatory markers. Moreover, although global perceived health is undoubtedly influenced by multiple factors including perceived mental health, bodily pain, fatigue, and physical functioning, no studies have examined the extent to which such aspects of self-rated health may drive the link between global health perceptions and inflammation.
The current study sought to replicate and extend upon previous findings to: 1) examine associations between a single-item measure of global self-rated health and serum inflammatory markers in older adults; 2) examine the relative strength of association of global self-rated health versus self-rated changes in recent health with inflammatory markers; 3) examine components of self-rated health that may underlie the association between inflammation and global self-rated health.
Method
Participants
Participants were part of a larger project on stress and health in older adults. They were recruited via notices placed in community and university newspapers, senior citizen centers, a collaborating neurologist, as well as through the Alzheimer’s Disease Association. Thus, the 250 participants included 107 older adults who were currently providing at least 5 hours per week of care for a spouse with Alzheimer’s disease or another progressive dementia, and 143 adults who were demographically indistinguishable from caregivers but had no similar caregiving responsibilities. Each participant completed a single study visit in which he or she provided a blood sample and completed questionnaires assessing perceived health, health conditions, health behaviors, and psychological factors.
At the time of recruitment, those reporting health problems with immunological consequences including cancer or recent surgeries were excluded as were those taking medications with broad immunological consequences. Participants were contacted by phone the day prior to the study visit. Any participant who reported acute illness including cold or flu-like symptoms within the previous two weeks was rescheduled. Data were collected between September 2004 and August 2009. The Ohio State University Biomedical Research Review Committee approved the project; all subjects gave written informed consent before participation.
Measurement of Peripheral Blood Interleukin-6 and C-Reactive Protein
All blood samples were drawn between 8AM and 10AM to control for diurnal variation. Serum interleukin (IL)-6 and C-reactive protein (CRP) were assayed using Quantikine High Sensitivity Immunoassay kits (R&D) per kit instructions, as described elsewhere (Glaser et al. 2003; Kiecolt-Glaser et al. 2003). These markers were chosen as a focus due to their associations with mortality (e.g.,Harris, Ferrucci et al. 1999). Samples were run undiluted in duplicate. Samples that fell out of range of the standard curve were retested, diluted 1:10 with diluent buffer included with the kit. Plates were read at a wavelength of 490nm with a correction wavelength of 690nm using a Multiscan MCC/340 plate reader (Labsystems). Sample concentrations were then extrapolated from a standard curve calculated using a four parameter logistic fit and then multiplied by the dilution factor if necessary. For CRP the intra- and inter-assay coefficients of variation were 5.1% and 7.3%, respectively. For IL-6, these values were 7.4% and 7.8%, respectively.
Health Conditions
Questions from the OARS Multidimensional Functional Assessment Questionnaire were used to assess problems including hypertension, thyroid problems, heart disease, diabetes, cancer, asthma, arthritis, emphysema, stroke, kidney disease, and liver disease and medications for each (Fillenbaum and Smyer 1981). The format is similar to those used in epidemiological studies (Berkman and Breslow 1983; Berkman et al. 1986), and provides a simple way to look at frequency of chronic conditions and medications.
Self-Rated Health
Self-rated health was assessed using the 36-item RAND Health Survey (Ware and Sherbourne 1992). Developed for the Medical Outcomes Study, this is a non-disease specific measure of functioning and well-being with excellent normative data (Ware and Sherbourne 1992). It includes two single-item measures which assess global self-rated health and perceived change in health in the past year. The global self-rated health question asked, “In general, would you say your health is: Excellent, Very Good, Good, Fair, or Poor”. The health change question asked, “Compared to one year ago, how would you rate your health in general now?” with five response options from “Much better now than one year ago” to “Much worse now than one year ago”. The RAND Health Survey also provides eight component scores: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, general mental health, social functioning, energy/fatigue, and general health perceptions. All items were coded so that higher scores indicated better health.
Depressive Symptoms
The Center for Epidemiological Studies Depression Scale (CES-D) was used to assess depressive symptomatology (Radloff 1977; Basco et al. 1997). Widely used and well-validated, the CES-D is a 20-item measure assessing cognitive, emotional, and somatic symptoms of depression (Basco, Krebaum et al. 1997).
Neuroticism
The 12-item Neuroticism scale of the Revised NEO Personality Inventory (NEO PI-R) was used to assess neuroticism. The NEO PI has been extensively studied and has strong psychometric properties (Costa and MccCrae 1992). Typified by a tendency to experience negative and distressing emotions, greater neuroticism has been associated with increased physical symptom reporting (Costa and McCrae 1987).
Health Behaviors
The Pittsburgh Sleep Quality Index (PSQI), a self-rated questionnaire, provides data on sleep quality and disturbances over a one-month interval (Buysse et al. 1989). The PSQI has good diagnostic sensitivity and specificity in distinguishing good and poor sleepers. Information regarding smoking behavior was collected via interview. Participants were categorized as never smokers (n=84), past social smokers (n=73), past regular smokers (n=101), and current smokers (n=17). Social smoking was defined by less than daily use and included those who tried cigarettes as teenagers but were never regular smokers. The Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire assessed the weekly frequency and duration of various physical activities. Excellent for middle-aged and older populations, it is a well-validated instrument (Stewart et al. 2001). We used the caloric expenditure variable, which estimates the total caloric expenditure per week based on the frequency, duration, and intensity of physical activities.
Physical Measurements
To control for effects of adiposity on outcomes of interest, body mass index (BMI) was calculated (kg/m2) using height and weight as measured by a nurse at the study visit.
Statistical Analyses
Multivariate linear regression models were fit to both log10 IL-6 and log10 CRP with self-rated health as the independent variable. Self-rated health was included as a continuous variable on the basis of evaluating residuals for each model. Residuals were plotted against predicted values to assess model assumptions of normality and independence. The primary model (Model I) controlled for age, gender, BMI, and total number of physical health conditions. Pearson correlation coefficients were estimated for each of these variables with IL-6 and CRP to describe and assess univariate associations. A significance level of α=0.05 was used for all tests.
We examined the effect of including individual diagnoses in place of total number of comorbidities. Because results were consistent whether number of comorbidities or specific comorbidities were used as control variables, number of comorbidities was retained as a control variable in subsequent models. In addition, all IL-6 and CRP measures were within 3 SD of the mean (log10 scale), so extreme cases were not a factor in the results.
As depressive symptoms may affect subjective ratings of health, we next tested a model which included control variables in Model I and also controlled for depressive symptoms, as assessed by the CES-D (Model II). Similarly, because neuroticism is associated with increased somatic complaints (Costa and McCrae 1987), we tested a model which included control variables in Model I and also controlled for neuroticism as assessed by the NEO PI-R. To examine the relative strength of the association of global self-rated health versus perceived change in health status over the past year with inflammatory markers, we included the same control variables as in Model I with the addition of self-reported change in health from the previous year (Model III).
Sensitivity analyses were performed to evaluate the stability and consistency of results from Models I, II, and III while accounting for health behaviors (sleep, smoking, and physical activity), medication usage, and caregiving status. For health behavior and medication usage, a risk factor modeling approach was taken, assessing the effect of each variable on the associations of self-rated health with IL-6 and CRP in each model. A variable was considered a confounder if inclusion in the model resulted in a change of >15% in one of the model coefficients or resulted in a non-significant association between IL-6 and CRP. The effect of caregiver status was evaluated by examining correlations with IL-6 and CRP within caregivers and controls separately, and by including caregiver status and the self-rated health by caregiver interaction in models I, II, and III. In addition to these analyses, caregiving status and gender were tested as potential effect modifiers; a variable was considered a modifier if there was a significant interaction between that variable and self-rated health in predicting IL-6 or CRP.
To examine the extent to which components of self-rated health were associated with inflammatory markers, we evaluated the association of each of the eight RAND subscales with IL-6 and CRP. Linear regression models utilizing the same control variables as Model I (age, BMI, gender, and number of health conditions) were fit using each subscale separately. Variables significant in these univariate models (univariate subscale plus controls) were combined into a multivariate linear regression model. Subscales with p-values greater than 0.05 were sequentially removed, beginning with the highest p-value, until all remaining subscale variables were significantly associated with the outcome.
Results
Health and Demographic Characteristics
Demographic and health characteristics are summarized in Table 1. The sample included 65 men and 185 women with an average age of 63.8 years (SD = 13.7), the majority were Caucasian/White (n=214, 85.6%) and highly educated, with 64% having an undergraduate degree or greater. Reflecting the relatively good health of the sample, 76% reported one or no health diagnoses and no participant endorsed “Poor” global self-rated health. Diagnoses, in order of frequency, were hypertension (n=90), thyroid problems (n=40), heart disease (n=33), diabetes (n=21), asthma (n=16), history of cancer (n=13), arthritis (n=10), emphysema (n=4), stroke (n=4), kidney disease (n=4), and liver disease (n=1). The mean serum IL-6 level (log10 scale) was 0.43 (SD =.018) pg/ml with a range of 0.12-1.04; the mean untransformed value for IL-6 was 1.99 (SD = 1.58) with a range of 0.32-10.00 pg/ml. The mean CRP level (log10scale) was 0.31 (SD = 0.50) mg/L with a range of −0.52-1.61; the mean untransformed value for CRP was 3.95 (SD = 5.32) mg/L with a range of 0.30-40.90. Correlational analyses demonstrated that global self-rated health was negatively associated with body mass index (BMI), number of health conditions, depressive symptoms, and sleep disturbance and positively associated with caloric expenditure (Table 2).
Table 1. Demographic and Psychosocial Characteristics.
| Characteristic | mean (SD) or n (%) |
|---|---|
| Age | 63.8 (13.7) |
| Gender | Female = 185 (74.0%) Male = 65 (26.0%) |
| BMI | 28.3 (6.3) |
| Race | Caucasian/White = 214 (85.6%) African-American/Black = 27 (10.8%) Asian = 4 (1.6%) Multi-racial =5 (2.0%) |
| Education | Less than high school = 2 (0.8%) High School = 32 (12.8%) Some college =56 (22.4%) College graduate = 96 (38.4%) Graduate or professional training = 64 (25.6%) |
| Caregiver status | Caregiver = 107 (42.8%) Noncaregiver = 143 (57.2%) |
|
Number of physical
health diagnoses |
None = 97 (38.8%) 1 = 93 (37.2%) 2 =43 (17.3%) 3 = 11 (4.4%) 4 = 6 (2.4%) |
| CES-D Scores | 8.7 (8.4) |
| Self-rated health | Excellent = 41 (16.4%) Very Good = 108 (43.2%) Good = 83 (33.2%) Fair = 18 (7.2%) Poor = 0 (0.0%) |
|
Change in health from
one year ago |
Much Better = 15 (6.0%) Somewhat better = 33 (13.3%) About the same = 164 (65.9%) Somewhat worse = 37 (14.9%) Much worse = 1 (0.4%) |
Table 2. Pearsona Correlations (r) Among Variables.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12.a | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Lg IL-6 | --- | .48*** | −.33*** | −.07 | .16* | .31*** | .23*** | .05 | .04 | .12 | −.02 | .08 |
| 2. Lg CRP | --- | −.28*** | −.06 | −.07 | .42*** | .14* | .10 | .07 | .15* | −.03 | −.05 | |
| 3. Global Self-Rated Health |
--- | .19** | .11 | −.26*** | −.27*** | −.31*** | −.30*** | −.33*** | .15* | −.15* | ||
| 4. Self-Rated Change in Health |
--- | −.13* | −.03 | −.09 | −.17** | −.06 | −.21*** | .21*** | .02 | |||
| 5. Age | --- | −.20** | .34*** | −.16* | −.16* | −.07 | −.06 | −.04 | ||||
| 6. BMI | --- | .11 | .16* | .04 | .19** | .05 | −.10 | |||||
| 7. # of Health Conditions |
--- | .05 | .03 | .13* | .07 | .05 | ||||||
| 8. Depressive Symptoms |
--- | .60*** | .59*** | −.05 | .11 | |||||||
| 9. Neuroticism | --- | .39*** | −.07 | .03 | ||||||||
| 10. Sleep Disturbance |
--- | −.05 | .09 | |||||||||
| 11. Caloric Expend per Week |
--- | −.00 | ||||||||||
| 12. Smoking Statusa |
--- |
With the exception of Smoking Status, which is reported as Spearman Correlations (rs)
p ≤ .05
p ≤.01
p ≤ .001. Lg = log10; higher health ratings correspond to better health
Aim 1: Associations between self-rated health and serum inflammatory markers.
As shown in Table 2, correlation analyses demonstrated that poorer self-rated health was associated with significantly higher IL-6 (r = −.33, p < .001; Figure 1) and CRP (r = −.28, p < .001; Figure 2). Linear regression controlling for age, gender, BMI and number of health conditions demonstrated that effects of self-rated health remained after controlling for these factors (Model I, Table 3). Although number of health conditions was significantly associated with both IL-6 and CRP, it was no longer significantly associated with either marker in multivariate regression analyses (Model I).
Figure 1.
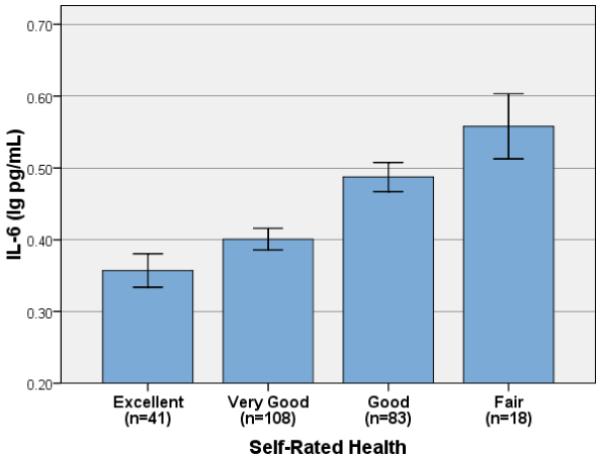
Mean Serum Interleukin-6 and Self-Rated Health (Error Bars: ± 1 SE)
Figure 2.
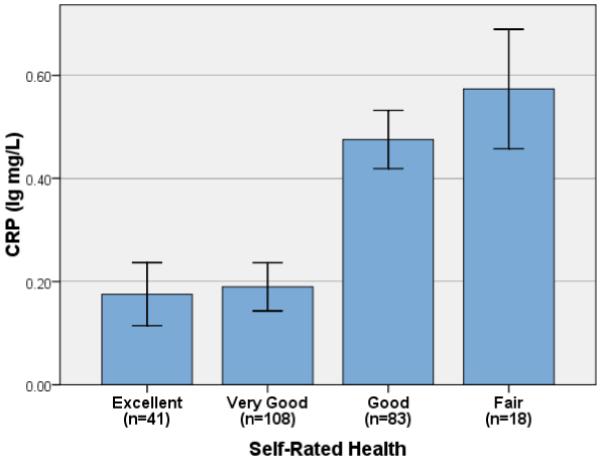
Mean Serum C-Reactive Protein and Self-Rated Health (Error Bars: ± 1 SE)
Table 3. Self-Rated Health as a Predictor of Serum IL-6 and CRP.
| IL-6 (lg pg/mL) | CRP (lg mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B (SE) | β | R2 | Δ R2 | t (246) | B (SE) | β | R2 | Δ R2 | t (246) | |
| Model I a | ||||||||||
| SRH | 0.05(.01) | 0.26 | 0.23 | 0.05 | 4.08*** | 0.07(.04) | 0.12 | 0.25 | 0.01 | 2.01* |
| Age | 0.003(.001) | 0.23 | 0.23 | 0.04 | 3.72*** | 0.001(.002) | 0.03 | 0.25 | 0.001 | 0.56 |
| BMI | 0.01(.002) | 0.29 | 0.23 | 0.07 | 4.81*** | 0.03(.005) | 0.39 | 0.25 | 0.13 | 6.55*** |
| Sex (F v M) | 0.03(.02) | 0.06 | 0.23 | 0.004 | 1.08 | 0.23(.07) | 0.20 | 0.25 | 0.04 | 3.42*** |
| Num Cond | 0.01(.01) | 0.05 | 0.23 | 0.003 | 0.85 | 0.03(.03) | 0.06 | 0.25 | 0.004 | 1.00 |
| Model II b | ||||||||||
| SRH | 0.06(.01) | 0.27 | 0.23 | 0.06 | 4.15*** | 0.08(.04) | 0.13 | 0.25 | 0.01 | 2.03* |
| Age | 0.003(.001) | 0.23 | 0.23 | 0.04 | 3.60*** | 0.001 (.002) | 0.03 | 0.25 | 0.02 | 0.51 |
| BMI | 0.01(.002) | 0.29 | 0.23 | 0.08 | 4.85*** | 0.03 (.005) | 0.39 | 0.25 | 0.13 | 6.55*** |
| Sex (F v M) | 0.03(.02) | 0.06 | 0.23 | 0.004 | 1.10 | 0.23 (.07) | 0.20 | 0.25 | 0.04 | 3.42*** |
| Num Cond | 0.01(.01) | 0.06 | 0.23 | 0.003 | 0.86 | 0.03 (.03) | 0.06 | 0.25 | 0.003 | 1.10 |
| CES-D | −0.001(.001) | −0.05 | 0.23 | 0.002 | −0.84 | −0.001 (.003) | −0.02 | 0.25 | 0.000 | −0.37 |
| Model III c | ||||||||||
| SRH | 0.06(.01) | 0.26 | 0.23 | 0.05 | 4.07*** | 0.07(.04) | 0.12 | 0.25 | 0.01 | 1.98* |
| Age | 0.003(.001) | 0.24 | 0.23 | 0.04 | 3.73*** | 0.001(.002) | 0.03 | 0.25 | 0.001 | 0.53 |
| BMI | 0.01(.002) | 0.29 | 0.23 | 0.11 | 4.81*** | 0.03(.005) | 0.39 | 0.25 | 0.13 | 6.53*** |
| Sex (F v M) | 0.03(.02) | 0.06 | 0.23 | 0.004 | 1.07 | 0.23(.07) | 0.20 | 0.25 | 0.04 | 3.41*** |
| Num Cond | 0.01(.01) | 0.05 | 0.23 | 0.002 | 0.84 | 0.03 (.03) | 0.06 | 0.25 | 0.004 | 1.01 |
| 1 Yr Change | 0.01(.01) | 0.02 | 0.23 | 0.000 | 0.41 | −0.005(.04) | −0.01 | 0.25 | 0.000 | −0.13 |
Model I Controlling for age, BMI, sex, and number of health conditions (Num Cond)
bModel II: Controlling for age, BMI, sex, number of health conditions, and CES-D scores
Model III: Controlling for age, BMI, sex, number of health conditions, and self-rated change in health in the previous year.
p ≤ 0.05
p ≤ 0.001
Further analyses demonstrated that these effects were not accounted for by differences in depressive symptoms (Model II, see Table 3). Although CES-D scores were significantly correlated with self-rated health (r = −.31, p <.001), CES-D scores were not significantly correlated with IL-6 or CRP (Table 2). Effects were also not explained by neuroticism. The addition of neuroticism to model I did not affect the association of self-rated health with IL-6 [B(SE)=0.05 (.01), β=0.25, t(1)=3.72, p<.001]. There were 19 subjects with missing neuroticism values which were excluded from analysis. Without these 19 subjects, the p-value for the self-rated health association with CRP in model I increased to p=0.07. With the addition of neuroticism, this association was only slightly affected [B(SE) = 0.07 (.04), β=0.11, t(1)=1.71,p=.09]. In these models neuroticism was associated with neither IL-6 [β=−0.01, t(1)=−0.17, p=.87] nor CRP [β=0.01, t(1)=0.14, p=.89].
Aim 2: Relative strength of association of perceived health versus perceived change in recent health with inflammatory markers.
Perceived change in health in the past year was not significantly correlated with IL-6 or CRP (Table 2). In addition, when included as a predictor in regression analyses (Model I + self-reported change in health), self-reported change in health from the previous year did not change the association between global self-rated health and either CRP or IL-6 (Model III, Table 3).
Aim 3: Components of self-rated health and serum inflammatory markers.
Regression analyses controlling for age, BMI, gender, and number of health conditions were run using IL-6 or CRP as the dependent variable and each of the eight component scales of the RAND as the predictor in separate regressions. Results demonstrated that the only subscale significantly related to inflammatory markers was physical functioning for IL-6 (B=−.01 (.004), t(248)=2.74, p = .01). A similar effect did not reach traditional statistical significance for CRP (B=−.03 (.01), t(248)=1.91, p = .06). Further analyses demonstrated that global self-rated health remained significantly associated with both IL-6 and CRP after controlling for perceived physical functioning.
Sensitivity Analyses
Potential confounding effects of medication usage and health behaviors were evaluated based on their effect on the association of self-rated health with IL-6 and CRP. A variable was considered a potential confounder if it resulted in a change of ≥ 15% in the self-rated health coefficient (Hosmer and Lemeshow 2000; Hosmer et al. 2008). The medications most commonly endorsed (by n=≥ 10 participants) were antihypertensives (n=80), antidepressants (n=51), thyroid medications (n=36), anticoagulants (n=32), heart disease medications (n=26), anxiety disorder medications (n=22), sedatives (n=21), and diabetes medications (n=19). Each of these medications was included individually into models I, II, and III for both IL-6 and CRP. The single-item global self-rated health measure remained significantly associated with IL-6 and CRP in all models and the self-rated health coefficient did not change by more than 15% in any of these models. Sleep, physical activity, and smoking were also added to all models and did not act as confounders for any models; specifically, for model III, the self-rated health coefficients changed by 4.5% (IL-6) and −4.9% (CRP) by inclusion of physical activity, 2.7% (IL-6) and 5.5% (CRP) by inclusion of smoking, and 2.3% (IL-6) and −0.5% (CRP) by inclusion of sleep quality. In addition, the effect of including education level in the model was tested; for model III, this resulted in a change of −1.7% (IL-6) and −5.8% (CRP) to the self-rated health coefficient and thus did not appreciably affect the relationship between self-rated health and serum inflammatory markers.
The consistency of associations between self-rated health and inflammatory markers among caregivers versus control participants was also examined. Caregivers and control participants were not statistically different in age (t(248) =0.91, p =0.36), gender (χ2(1) = 0.84, p =.89), BMI (t(248) = −0.94, p =.35), or education (t(248) = 0.526, p =0.60). Caregivers reported a greater total number of physical health conditions; [mean (SD)=1.12 (1.06) versus 0.85 (0.07), t(248)=3.90, p <.001], greater depressive symptoms; [11.02 (8.2) versus 6.97 (8.05), t(248) = 3.90, p <.001], and poorer self-rated health [2.47 (0.81) versus 2.20 (0.83), t(248) = 0.01]. Caregivers and controls did not differ in serum levels of IL-6 (t(248) = 0.25, p = 0.81) or CRP (t(247) =−0.95, p =0.34).
Separate correlation analyses demonstrated that negative associations between self-rated health and IL-6 and CRP were present and did not differ significantly for caregivers and controls (difference in Pearson’s r: IL-6, p = .67; CRP, p = .94). For caregivers, better self-rated health was associated with significantly lower IL-6 (r = −.31, p =.001) and lower CRP (r = −.27, p =.005). For controls, the same associations were found for both IL-6 (r = −.36, p <.001) and CRP (r = −.26, p =.002). Further, inclusion of caregiving status in the models did not significantly alter the relationship between self-rated health and CRP or IL-6: self-rated health remained significantly associated with both (F(1,241) = 3.76, p=.05) and IL-6 (F(1,242) = 17.88, p<.001) when included in model III. Further, the interaction of self-rated health and caregiving status was not significant for either CRP or IL-6; when included in model III, the caregiver X self-rated health interaction term was F(1,242) = 1.12, p = .29 for IL-6 and F(1,241) = .44, p = .51 for CRP.
Discussion
Single-item measures of self-rated health have proven to be robust and independent predictors of mortality in numerous and demographically diverse samples (for review see Idler and Benyamini 1997; Benyamini and Idler 1999). Inflammatory processes may play a key role in this link; inflammation promotes a variety of chronic diseases and is well-known to induce symptoms of sickness behavior, as detailed below. Such general symptoms may be perceived by an individual in the absence of a diagnosable disease and/or well before a specific disease is detectable by objective measures. The current study supports the hypothesis that inflammatory processes are associated with perceptions of self-rated health. Among this sample of 250 relatively healthy older adults, lower ratings on a single-item measure of perceived health were associated with higher serum IL-6 and CRP. This effect was not accounted for by objective health diagnoses, BMI, age, gender, depressive symptoms, neuroticism or health behaviors, suggesting a role for more direct physiological pathways.
It is now well-established that proinflammatory cytokines induce sickness behavior (Dantzer 2001). Sickness behavior is typified by non-specific symptoms of illness, including weakness, difficulty concentrating, fatigue, lack of appetite, and lack of interest. Cytokines act directly on the brain to produce these behavioral and perceptual changes. The most rapid pathway by which peripheral cytokines affect the brain is via activation of afferent vagal nerve fibers that innervate organs of the abdominal cavity (Konsman et al. 2002). Via this pathway, peripheral cytokines can induce the synthesis and release of proinflammatory cytokines in the brain (Dantzer 2001). In addition, although transfer of cytokines from peripheral circulation to the brain is largely prevented by the blood-brain-barrier, cytokines can enter the brain via weaker areas of the blood-brain-barrier as well as through active cytokine transporters (Raison et al. 2006). Importantly, cytokine receptors are located throughout the brain including the hypothalamus and hippocampus, which are key regulators of sickness behaviors (Konsman et al. 2002; Bailey et al. 2003). The sickness response is highly adaptive in the context of illness, directing behavior and motivation in a manner that promotes rest and healing. However, such symptoms may certainly be perceived as uncomfortable and would be expected to reduce and one’s perception of current health.
It is notable that self-rated health was associated with serum inflammatory markers even among relatively healthy older adults. In this sample, 76% reported no or one health diagnosis. No participant endorsed “poor” health. In comparison, in population-based studies, 7-12% of community-dwelling older adults reported poor health (Wolinsky and Johnson 1992; Cohen, Pieper et al. 1997; Jylha et al. 2006). Thus, these data extend previous findings and provide strong support for the notion that associations of self-rated health with later health outcomes are not purely a function of a person’s knowledge of objective diagnoses.
Of note, although the generally healthy status of this sample provides the benefit of reducing problematic confounding of self-rated health with objective health diagnoses, the stringent health inclusion criteria for the study also limit the generalizability of these findings. Indeed, in addition to having excellent overall health, this sample of older adults was highly educated and primarily Caucasian. Replication and extension of these findings in larger and more diverse samples is warranted.
It has been suggested that the predictive power of self-rated health for mortality may be due, in part, to a self-reference effect whereby global health ratings actually reflect perceived declines in health, rather than simple assessments of current functioning (Idler and Benyamini 1997). This study demonstrated that perceived change in health was not associated with serum inflammatory markers. Further, associations between global self-rated health and inflammatory markers were not affected by inclusion of perceived change in health in the model. Thus, effects of self-rated health were not simply a function of perceived stability versus decrements in recent health. In future research with longitudinal design, it would be informative to examine the effects of change in global self-rated health across multiple years.
Global self-rated health is likely influenced by comparisons to age peers and others in one’s social network (Manderbacka et al. 2003). The current sample included adults who provide care for a spouse with Alzheimer’s or other progressive dementia, an experience that could reduce global self-rated health due to greater experience of negative emotions and fatigue (Schulz et al. 1995; Pinquart and Sorensen 2003; Lutgendorf and Laudenslager 2009; Roepke et al. 2009). In addition, the experience of caregiving could influence the reference point by which one rate’s his or her own health, altering the extent to which self-rated health provides predictive value for inflammation. Results demonstrated that, although caregivers reported poorer self-rated health than controls, inflammatory markers and self-rated health were significantly associated in both groups. Thus, the experience of caregiving did not alter perceptions of self-rated health in a manner that reduced its association with health outcomes.
As in previous studies (Cohen, Pieper et al. 1997; Jylha et al. 2002; Lekander, Elofsson et al. 2004; Jylha, Volpato et al. 2006), self-rated health was associated with inflammatory markers after controlling for physical health conditions, indicating that this relationship was not accounted for by knowledge of one’s objective health diagnoses. Indeed, although the number of physical health diagnoses was significantly correlated with both IL-6 and CRP, it was no longer significantly associated with either inflammatory marker in the full regression model which included age, BMI, gender, and self-rated health.
Health behaviors including smoking, physical activity, and sleep are key modifiable predictors of healthy aging as well as inflammatory markers (Peel et al. 2005; Okun et al. 2011). Thus, it is likely that knowledge of one’s own health behaviors influence subjective ratings of health. Indeed, the current data demonstrated that poorer self-rated health was associated with both poorer sleep and lower physical activity. However, controlling for these factors did not attenuate the association between self-rated health and serum inflammatory markers.
This study also examined associations between components of self-rated health as measured by the eight subscales of the RAND Health Survey and inflammatory markers. The physical functioning subscale was significantly associated with IL-6; this relationship did not reach statistical significance for CRP. Controlling for scores on the physical functioning subscale did not attenuate the relationship between the single-item global self-rated health measure and either IL-6 or CRP. Thus, the association of the single-item measure of global self-rated health with inflammatory markers was not explained by any particular facet of health, as measured by the RAND.
An important limitation of this study is that it is cross-sectional in design; thus the current data do not allow for determination of the causal direction or temporal associations between self-rated health and inflammatory markers. As described, one plausible causal direction is that inflammatory processes influence subjective ratings of health via cytokine-induced symptoms. It seems unlikely that subjective health ratings would directly exert an influence on cytokine levels. Our analyses demonstrated that depressive symptoms, neuroticism, and key health behaviors did not explain the association of self-rated health with inflammatory markers. Thus, our results are in concert with a direct physiological pathway linking self-rated health and inflammation (i.e., sickness behavior). However, the associations noted could also be attributable to unmeasured behavioral or personality factors. Future research of longitudinal design with inclusion of a greater number of potential causal mediators would help to elucidate these questions. However, regardless of the factors which ultimately contribute to the relationship between self-rated health and serum cytokine levels, the current data demonstrate that subjective health ratings are of clinical utility in predicting biomarkers relevant to health.
An important limitation of this study is that it did not include mortality data. Therefore, we were unable to examine the extent to which self-rated health and inflammatory markers may represent independent versus common predictive pathways to increased mortality risk. Longitudinal data linking self-rated health, inflammatory markers, and mortality risk within the same study would be highly informative.
In sum, self-rated health was associated with serum inflammatory markers among generally healthy older adults. The association between self-rated health and inflammation was not accounted for by objective health diagnoses, medication use, or health behaviors. This association was not due to effects of depressive symptoms and was not accounted for by perceived changes in recent health. Thus, self-rated health provides unique information regarding inflammatory status above and beyond traditional objective health indicators.
Acknowledgments
We appreciate the helpful assistance of Cathie Atkinson and Bryon Laskowski with the study, as well as the Central Ohio Alzheimer’s Association.
Role of the Funding Sources
The project described was supported by Award Number UL1RR025755 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. This research was also supported by NIH grants CA16058 (J.K-G.), AG025732 (J. K-G.), HD06144 (L.M.C) and T32AI55411 (L.M.C.). NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey M, Engler H, Hunzeker J, Sheridan JF. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol. 2003;16:141–157. doi: 10.1089/088282403322017884. [DOI] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. American Psychological Association; Washington D. C.: 1997. pp. 207–245. [Google Scholar]
- Benyamini Y, Blumstein T, Lusky A, Modan B. Gender differences in the self-rated health-mortality association: Is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? Gerontologist. 2003;43:396–405. doi: 10.1093/geront/43.3.396. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Idler EL. Community studies reporting association between self-rated health and mortality - Additional studies, 1995 to 1998. Res. Aging. 1999;21:392–401. [Google Scholar]
- Berkman LF, Berkman CS, Kasl S, Freeman DH, Leo L, Ostfeld AM, Cornoni-Huntley J, Brody JA. Depressive symptoms in relation to physical health and functioning in the elderly. Am. J. of Epidemiol. 1986;124:372–388. doi: 10.1093/oxfordjournals.aje.a114408. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Breslow L. Health and ways of living: The Alameda county study. Oxford University Press; New York: 1983. [Google Scholar]
- Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain. Behav. Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Siegler IC, Brummett BH, Barefoot JC, Williams RB, Clapp-Channing NE, Mark DB. The association between self-rated health and mortality in a well-characterized sample of coronary artery disease patients. Med. Care. 1999;37:1226–1236. doi: 10.1097/00005650-199912000-00006. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JVB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 2003;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr. Opin. Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J. Clin. Endocrinol. Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J. Gerontol. A. Biol. Sci. Med. Sci. 1997;52:M201–208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr., McCrae RR. Neuroticism, somatic complaints, and disease: is the bark worse than the bite? J. Pers. 1987;55:299–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Costa PT, MccCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five Factor Inventory (NEO-FFI): Professional manual. Psychological Assessment Resources; Odessa, Fla: 1992. [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. N. Y. Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behav., Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasbach EJ, Klein R, Klein BEK, Moss SE. Self-Rated Health and Mortality in People with Diabetes. Am. J. Public Health. 1994;84:1775–1779. doi: 10.2105/ajph.84.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Keller E. Age-associated increased interleukin-6 gene expression, late life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS Multidimensional Functional Assessment Questionnaire. J. Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- Franks P, Gold MR, Fiscella K. Sociodemographics, self-rated health, and mortality in the US. Soc. Sci. Med. 2003;56:2505–2514. doi: 10.1016/s0277-9536(02)00281-2. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles T, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses following influenza vaccination in older adults. Arch. Gen. Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Hamerman D, Berman JW, Albers GW, Brown DL, Silver D. Emerging evidence for inflammation in conditions frequently affecting older adults: Report of a symposium. J. Am. Geriatr. Soc. 1999;47:1016–1025. doi: 10.1111/j.1532-5415.1999.tb01299.x. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regresssion. John Wiley and Sons; New York: 2000. Chapter 4: Model-building strategies and methods for logistic regression. [Google Scholar]
- Hosmer DW, Lemeshow S, May S. Chapter 5: Model development Applied Survival Analysis. J. W. a. Sons; New Jersey: 2008. [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J. Health Soc. Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain Behavior and Immunity. 2005;19:555–563. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Jylha M, Volpato S, Guralnik J. Self-rated health is associated with biomarkers, but predicts mortality independently of them. Gerontologist. 2002;42:284–284. [Google Scholar]
- Jylha M, Volpato S, Guralnik JM. Self-rated health showed a graded association with frequently used biomarkers in a large population sample. J. Clin. Epidemiol. 2006;59:465–471. doi: 10.1016/j.jclinepi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Lekander M, Elofsson S, Neve I-M, Hansson L-O, Unden A-L. Self-rated Health Is Related to Levels of Circulating Cytokines. Psychosom. Med. 2004;66:559–563. doi: 10.1097/01.psy.0000130491.95823.94. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Laudenslager ML. Care of the caregiver: stress and dysregulation of inflammatory control in cancer caregivers. J. Clin. Oncol. 2009;27:2894–2895. doi: 10.1200/JCO.2009.22.1523. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: A magnificent pathway. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderbacka K, Kareholt I, Martikainen P, Lundberg O. The effect of point of reference on the association between self-rated health and mortality. Soc. Sci. Med. 2003;56:1447–1452. doi: 10.1016/s0277-9536(02)00141-7. [DOI] [PubMed] [Google Scholar]
- Okun ML, Reynolds CF, III, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall MP. Sleep Variability, Health-Related Practices, and Inflammatory Markers in a Community Dwelling Sample of Older Adults. Psychosom. Med. 2011;73:142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Bruunsgaard H, Ostrowski K, Krabbe K, Hansen H, Krzywkowski K, Toft A, Sondergaard SR, Petersen EW, Ibfelt T, Schjerling P. Cytokines in aging and exercise. Int. J. Sports Med. 2000;21:S4–S9. doi: 10.1055/s-2000-1444. [DOI] [PubMed] [Google Scholar]
- Peel NM, McClure RJ, Bartlett HP. Behavioral determinants of healthy aging. Am. J. Prev. Med. 2005;28:298–304. doi: 10.1016/j.amepre.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sorensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: A meta-analysis. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2003;58:P112–P128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke SK, Mausbach BT, von Kanel R, Ancoli-Israel S, Harmell AL, Dimsdale JE, Aschbacher K, Mills PJ, Patterson TL, Grant I. The moderating role of personal mastery on the relationship between caregiving status and multiple dimensions of fatigue. Int. J. Geriatr. Psychiatry. 2009;24:1453–1462. doi: 10.1002/gps.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubenoff R, Parise H, Payette HA, Abad LW, D’Agostino R, Jacques PF, Wilson PWF, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. Am. J. Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Schulz R, Obrien AT, Bookwala J, Fleissner K. Psychiatric and Physical Morbidity Effects of Dementia Caregiving - Prevalence, Correlates, and Causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Gueguen A, Martikainen P, Ferrie J, Marmot M, Shipley M. Self-rated health and mortality: Short- and long-term associations in the Whitehall II study. Psychosom. Med. 2007;69:138–143. doi: 10.1097/PSY.0b013e318030483a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med. Sci. Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Thomas C, Kelman HR, Kennedy GJ, Ahn C, Yang CY. Depressive Symptoms and Mortality in Elderly Persons. J of Gerontol. 1992;47:S80–S87. doi: 10.1093/geronj/47.2.s80. [DOI] [PubMed] [Google Scholar]
- Thong MSY, Kaptein AA, Benyamini Y, Krediet RT, Boeschoten EW, Dekker FW, NECOSAD Association between a self-rated health question and mortality in young and old dialysis patients: A cohort study. Am. J. Kidney Dis. 2008;52:111–117. doi: 10.1053/j.ajkd.2008.04.001. A. D. [DOI] [PubMed] [Google Scholar]
- Unden AL, Andreasson A, Elofsson S, Brismar K, Mathsson L, Ronnelid J, Lekander M. Inflammatory cytokines, behaviour and age as determinants of self-rated health in women. Clin Sci (Lond) 2007;112:363–373. doi: 10.1042/CS20060128. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-Item-Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Wolinsky FD, Callahan CM, Fitzgerald JF, Johnson RJ. Changes in Functional Status and the Risks of Subsequent Nursing-Home Placement and Death. J. Gerontol. 1993;48:S93–S101. [PubMed] [Google Scholar]
- Wolinsky FD, Johnson RJ. Perceived health status and mortality among older men and women. J. Gerontol. 1992;47:S304–312. doi: 10.1093/geronj/47.6.s304. [DOI] [PubMed] [Google Scholar]
- Yao L, Robert SA. The contributions of race, individual socioeconomic status, and Neighborhood socioeconomic context on the self-rated health trajectories and mortality of older adults. Res. Aging. 2008;30:251–273. [Google Scholar]


