Abstract
OBJECTIVE
Because direct adipose tissue free fatty acid (FFA) storage may contribute to body fat distribution, we measured FFA (palmitate) storage rates and fatty acid (FA) storage enzymes/proteins in omental and abdominal subcutaneous fat.
RESEARCH DESIGN AND METHODS
Elective surgery patients received a bolus of [1-14C]palmitate followed by omental and abdominal subcutaneous fat biopsies to measure direct FFA storage. Long chain acyl-CoA synthetase (ACS) and diacylglycerol acyltransferase activities, CD36, fatty acid-binding protein, and fatty acid transport protein 1 were measured.
RESULTS
Palmitate tracer storage (dpm/g adipose lipid) and calculated palmitate storage rates were greater in omental than abdominal subcutaneous fat in women (1.2 ± 0.8 vs. 0.7 ± 0.4 μmol ⋅ kg adipose lipid−1 ⋅ min−1, P = 0.005) and men (0.7 ± 0.2 vs. 0.2 ± 0.1, P < 0.001), and both were greater in women than men (P < 0.0001). Abdominal subcutaneous adipose tissue palmitate storage rates correlated with ACS activity (women: r = 0.66, P = 0.001; men: r = 0.70, P = 0.007); in men, CD36 was also independently related to palmitate storage rates. The content/activity of FA storage enzymes/proteins in omental fat was dramatically lower in those with more visceral fat. In women, only omental palmitate storage rates were correlated (r = 0.54, P = 0.03) with ACS activity.
CONCLUSIONS
Some adipocyte FA storage factors correlate with direct FFA storage, but sex differences in this process in visceral fat do not account for sex differences in visceral fatness. The reduced storage proteins in those with greater visceral fat suggest that the storage factors we measured are not a predominant cause of visceral adipose tissue accumulation.
Excess visceral fat is associated with greater metabolic risk (1,2), whereas preferential lower body fat accumulation is not (3). The mechanisms by which some individuals gain fat in one depot at the expense of another are unknown, but surely relate to an imbalance between storage and release of fatty acids (FAs). The patterns of regional free FA (FFA) release suggest that lipolysis defects cannot explain adipose tissue distribution patterns (4–6). Likewise, meal fat storage patterns do not completely explain regional variations in adipose tissue accumulation (7–11).
The direct FFA storage pathway, which is lipoprotein lipase (LPL) independent, exists in both animal (12) and human (13–15) adipose tissue in vivo. Furthermore, qualitative patterns of postabsorptive, direct adipose tissue FFA storage mirror that of body fat distribution (14). Direct FFA storage in men, but not women, is greater in the upper body than lower body subcutaneous fat, and women store FFA more efficiently in subcutaneous fat than men. Hannukainen et al. (16), using positron emission tomography scan technology, found that FFA storage rates are greater in visceral than subcutaneous fat in nonobese men, similar to what we observed in nonobese women (15).
Because the direct FFA storage pathway seems to help determine body fat distribution, gaining insight into the adipocyte factors regulating FA incorporation into triacylglycerols may help explain depot differences in fat storage. Potential adipocyte specific rate-limiting steps include 1) facilitated FA transport across the plasma membrane, mediated by proteins such as CD36, plasma membrane-associated fatty acid-binding protein (FABP[pm]), and fatty acid transport protein 1 (FATP1); 2) acylation, which leads to activation/trapping of intracellular FAs, mediated by the activity of a number of fatty acyl-CoA synthetases (ACSs); and 3) the final step of triacylglycerol formation, mediated by diacylglycerol acyltransferase (DGAT).
In this study, we measured direct FFA storage into visceral and abdominal subcutaneous adipose tissue using isotope tracer/adipose biopsy techniques. Our hypothesis was that men, typically having more visceral fat than women, would have greater direct FFA storage in omental than abdominal subcutaneous adipose tissue and greater direct omental FFA storage than women. We also measured factors related to the FA storage steps described above and studied their relation to direct FFA storage into adipocyte triglyceride. We hypothesized that depot differences in these factors would correlate with regional, sex-specific FFA storage differences. The results provide evidence for major between-depot and between-individual differences in the FA storage factors that relate to direct FFA storage.
RESEARCH DESIGN AND METHODS
Subjects.
The study was approved by the institutional review boards of the Mayo Clinic and Ersta Hospital, Stockholm, Sweden. Written, informed consent was obtained from all study participants. Patients were included if 1) their BMI was ≤35 kg/m2; 2) they were not taking any medications that could affect lipid metabolism or adiposity; 3) their liver and kidney function studies were normal; and 4) they had no systemic inflammatory illnesses. The participants recruited at Ersta Hospital were undergoing elective cholecystectomy for uncomplicated gallstones, and those at Mayo Clinic were undergoing donor nephrectomy.
Body composition.
Body fat, fat free mass, and regional fat mass were measured using dual-energy X-ray absorptiometry (DXA). The Mayo patients underwent a preoperative abdominal computed tomography (CT) scan for clinical purposes, and images from the L2–3 interspace were used in combination with regional abdominal DXA data to determine visceral fat mass. Patients at Ersta Hospital underwent a single-slice abdominal CT at the L2–3 interspace. Visceral fat mass was subtracted from regional abdominal fat mass determined by DXA to determine abdominal subcutaneous fat mass. Four of the Mayo patients were unable to have a DXA scan performed before surgery, and we therefore measured abdominal subcutaneous fat and visceral fat using all of the CT images from the dome of the diaphragm to the superior aspect of the greater trochanter (17).
Protocol.
Patients consumed their usual diet until the day of admission and reported to the hospital the morning of the surgery. On arrival in the operating room, a baseline blood sample was collected. An intravenous bolus of ∼60 μCi of albumin bound [1-14C]palmitate (18) was given before induction of anesthesia and ∼30 min before the collection of adipose tissue samples. The exact tracer dose was determined by counting four 50-μL aliquots. The tracer bolus was timed such that adipose samples would be collected 30–45 min later, an interval during which virtually all FFA tracer is cleared from the circulation and significant incorporation of the tracer into VLDL-triglyceride has yet to occur (14). Excisional biopsies of abdominal subcutaneous and omental adipose tissue were generally performed within 5 min of each other. Portions of the samples were flash-frozen for measurement of the FA storage enzyme activities and proteins (see below) or placed in HEPES solution for transport to our laboratory, where the adipocytes were isolated by collagenase digestion to measure adipocyte lipid specific activity (SA) and to prepare the adipocyte plasma membrane fraction for FABP(pm) analysis.
Adipose tissue handling.
Adipocytes were isolated by collagenase digestion, and lipid was extracted (to avoid contamination from extracellular FA). Adipocyte lipid SA was determined as previously described (14). For protein content and enzyme assays, ∼500 mg of the flash-frozen adipose tissue was homogenized in 2 mL of Standard Homogenization Buffer (SHB-P; 20 mmol/L Tris-HCl, pH 7.4, 1 mmol/L EDTA, 255 mmol/L sucrose) with antiprotease tablets (Roche Diagnostics Corporation, Indianapolis, IN). Supernatant was collected after centrifugation at 2,100 rpm at 4°C for 10 min. The lipid was quantitatively extracted (chloroform: methanol) and weighed so that we could normalize for protein content and enzyme activity per unit weight lipid.
Assays
Plasma FFA concentrations.
Plasma palmitate concentrations were measured using high-performance liquid chromatography (18).
CD36.
We used a sandwich enzyme-linked immunosorbent assay to measure adipose tissue CD36 content (19).
FATP1.
To prepare a crude membrane preparation, approximately 500 μL of the supernatant was topped off to 2 mL with SHB-P. This sample was ultracentrifuged at 37,500 rpm for 30 min. The resultant pellet was resuspended in ∼62.5 μL of SHB-P with 2% SDS and then went through two cycles of vortex, sonication, and incubation at 95°C for 5 min. This was then microcentrifuged for 10 min at full speed, and the supernatant was kept as the membrane sample. Membrane extracts were separated by SDS-PAGE and immunoblotted using affinity purified rabbit anti-FATP1 antiserum (20) and detected using horseradish peroxidase conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA).
FABP(pm).
Plasma membrane extracts were subjected to 12% SDS-PAGE, transferred onto Immobilin-P-polyvinylidene difluoride membranes and immunoblotted using affinity purified rabbit anti-FABP(pm) (21), and detected using horseradish peroxidase conjugated goat anti-rabbit IgG (Pierce, Rockford, IL). The membranes were developed via enhanced chemiluminescence (Pierce) followed by exposure to CL-XPosure film (Pierce). Membranes were stripped and reprobed with the glyceraldehyde-3-phosphate dehydrogenase antibody to confirm equal loading. The films were scanned using a Hewlett Packard ScanJet 6200C (Hewlett-Packard Co., Palo Alto, CA) and quantified using Scion Image (Scion, Frederick, MD). In all cases, multiple gels were analyzed and compared with results obtained in a control sample (L6 cell lysate) and protein content was normalized to glyceraldehyde-3-phosphate dehydrogenase as the housekeeping control protein. We found that >95% of adipocyte CD36 is in the plasma membrane (19). Because we also measured the CD36 content of adipocyte plasma membrane relative to membrane protein and to whole tissue extract protein and whole tissue lipid, we were able to calculate the adipocyte plasma membrane FABP(pm) content relative to tissue lipid to provide consistency in terms of data expression.
Fatty ACS.
We measured the conversion of [3H]palmitate to its CoA derivative using the method of Hall et al. (22). The intra- and interassay coefficient of variation in our laboratory is <10%.
DGAT.
We used the method of Coleman (23) modified slightly to use the cytosol fraction (24) of the adipose tissue homogenate, and we used 20 μL of 10.0 mmol/L (rather than 2.0 mmol/L) 1,2 Dioleoyl-sn-glycerol (Sigma D-0138 FW:621; Sigma-Aldrich, St. Louis, MO) in the reaction mixture.
mRNA.
RNA was extracted using Qiagen’s RNeasy lipid tissue mini kit (Qiagen Inc., Venlo, the Netherlands). A cDNA library was made using Applied Biosystem’s High Capacity cDNA Archive kit (Carlsbad, CA). Quantitative RT-PCR was performed on an ABI 7900 using primer and probe sets from Applied Biosystems (peroxisome proliferator-activated receptor [PPAR]-γ 1, A1T95DS [custom designed by Sewter et al. (25); PPAR-γ 2, Hs01115510_m1; CCAAT/enhancer-binding protein (C/EBP-α), Hs00269972_s1; CYCA, Hs99999904_m1]). Calculations of relative transcript amounts were normalized to a “housekeeping”/endogenous control gene (cyclophilin A) and then reported relative to a calibrator sample (surgical fat).
Calculations and statistics.
Values are provided as mean ± SEM unless otherwise stated. The storage of the palmitate tracer was assessed directly using the adipose tissue lipid SA (dpm/g adipocyte lipid) adjusted for the amount of tracer administered for each participant. Because these values are not normally distributed, statistical analysis of the adipose tissue lipid SA data was done on log-transformed data. The rates of palmitate storage in adipose tissue are calculated using the fractional storage of [14C]palmitate (dpm/g of adipocyte lipid ÷ dpm administered) in adipocyte lipid and the rate of palmitate disappearance. Given the logistics of tracer infusions and sample collection in the surgical suite, we used a regression formula to predict palmitate flux rather than using a separate tracer infusion. This formula was derived using two large independent datasets. We found that sex, age, BMI, and palmitate concentrations could predict palmitate flux with an R2 of 0.65 and confirmed that this formula could predict palmitate flux equally well when applied to other datasets generated in our laboratory. The calculated palmitate rate of disappearance was multiplied by the fractional [14C]palmitate storage in abdominal subcutaneous and omental adipose tissue to determine rates of palmitate storage (nmol ⋅ g adipose lipid−1 ⋅ min−1).
Paired t test was used for comparison of results between depots within the same individuals, and nonpaired t test was used for comparisons between men and women. Univariate regression analyses were used to test for correlations between palmitate storage and FA storage enzyme activity and protein content. Univariate regression analysis was also used to determine whether the FA storage proteins/enzyme activities were highly correlated.
RESULTS
Subject characteristics.
Men and women were not significantly different in age and BMI. The expected sex differences in body composition were present. Palmitate concentrations were not significantly different between women and men, but were approximately 50% greater (total FFA concentrations of ∼640 and ∼540 μmol/L, respectively) than the average overnight postabsorptive palmitate concentrations that are typically observed in a clinical research unit setting (Table 1).
TABLE 1.
Characteristics of subjects
| Women (n = 16) | Men (n = 14) | P value | |
|---|---|---|---|
| Age (years) | 47 ± 3 | 44 ± 4 | 0.6 |
| Weight (kg) | 73.1 ± 3 | 88.5 ± 3 | 0.003 |
| BMI (kg/m2) | 27.4 ± 1 | 26.9 ± 1 | 0.75 |
| % Body fat | 40 ± 2 | 25 ± 2 | <0.0001 |
| Fat mass (kg) | 30.4 ± 3 | 21.6 ± 3 | 0.04 |
| Fat free mass (kg) | 38.6 ± 3 | 59.7 ± 4 | 0.0002 |
| Abdominal subcutaneous fat (kg) | 7.5 ± 0.9 | 4.3 ± 0.9 | 0.02 |
| Visceral fat (kg) | 3.0 ± 0.6 | 4.7 ± 0.7 | 0.1 |
| Plasma palmitate (μmol/L) | 147 ± 50 | 124 ± 41 | 0.2 |
Values represent mean ± SEM. P values refer to comparisons between men and women.
Measures of direct FFA storage
Adipocyte lipid SA.
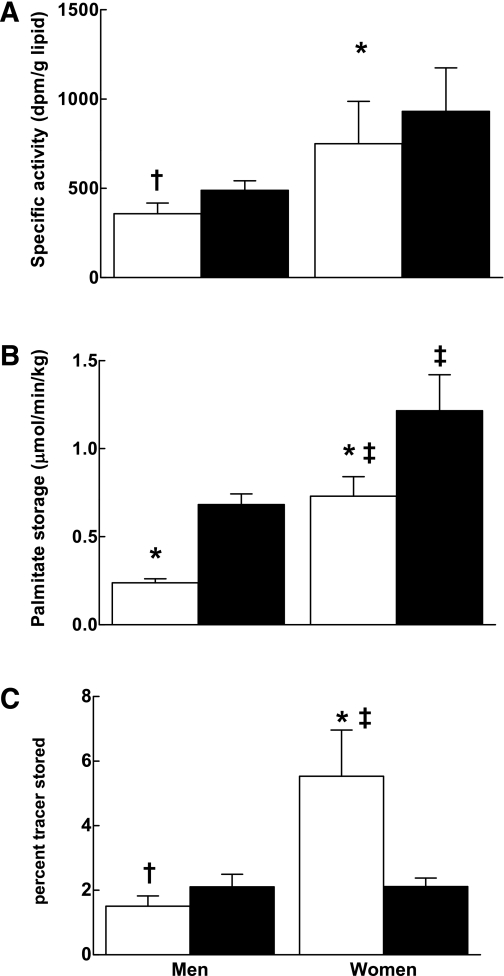
The adipocyte lipid SA adjusted for the tracer dose was greater in omental than abdominal subcutaneous fat in both men (489 ± 53 vs. 358 ± 60 dpm/g, P = 0.03) and women (931 ± 243 vs. 750 ± 237, P = 0.01). The adipocyte lipid SA was greater in women than men for both the omental (P < 0.05) and abdominal (P < 0.01) subcutaneous sites (Fig. 1A).
FIG. 1.
Comparison of regional difference in FA storage. A: SA (dpm/g lipid). B: FFA storage (μmol ⋅ kg lipid−1 ⋅ min−1). C: Percent storage. Open bars represent abdominal subcutaneous adipose tissue, and closed bars represent omental adipose tissue. †P < 0.05 vs. omental adipose tissue within same sex group. *P < 0.01 vs. omental adipose tissue within same sex group. ‡P < 0.03 vs. same depot in men.
Calculated palmitate storage rates.
Palmitate storage rates were greater in omental than abdominal subcutaneous adipose tissue in both women (1.2 ± 0.8 vs. 0.7 ± 0.4 μmol ⋅ kg lipid−1 ⋅ min−1, P = 0.005) and men (0.7 ± 0.2 vs. 0.2 ± 0.1, P < 0.001). Palmitate storage rates into both depots were significantly greater in women than in men (P < 0.0001). The relationship between omental palmitate storage rates and visceral fat mass in women was such that those with more visceral fat had reduced rates of storage (r = −0.48, P = 0.06), whereas omental adipose tissue palmitate storage rates in men did not vary as a function of visceral fat mass. Palmitate storage in abdominal subcutaneous fat did not vary as a function of fat mass (Fig. 1B).
Fractional storage (% tracer stored per depot).
Men stored a greater fraction of the palmitate tracer in the omental than the abdominal subcutaneous depot (2.1 ± 0.4 vs. 1.5 ± 0.3%, P = 0.05) (Fig. 1C). In contrast, women stored a greater fraction of tracer in abdominal subcutaneous than omental fat (5.5 ± 1.4 vs. 2.1 ± 0.3%, P = 0.02). The fraction of palmitate tracer stored in abdominal subcutaneous fat was greater in women than men (P = 0.02), but not different in omental fat.
Comparison of FA storage factors between abdominal subcutaneous and omental adipose tissue.
ACS and DGAT activity per milligram of lipid were significantly greater in omental than abdominal subcutaneous adipose tissue in women and men (all P < 0.05) (Table 2). FABP(pm) per milligram of lipid was significantly greater in omental than abdominal subcutaneous adipose tissue (P < 0.05) in women. Adipocyte CD36 and FATP1 content were not statistically different between men and women and between depots.
TABLE 2.
Adipose tissue enzyme activity and protein content
| Men |
Women |
|||
|---|---|---|---|---|
| Abdominal SQ | Omental | Abdominal SQ | Omental | |
| ACS activity (pmol/min/mg lipid) | 25 ± 5 | 106 ± 30† | 42 ± 8 | 107 ± 30† |
| DGAT activity (pmol/min/mg lipid) | 3 ± 0.6 | 13 ± 3† | 5 ± 1 | 12 ± 3† |
| CD36 (units/mg lipid) | 8 ± 1 | 12 ± 2 | 14 ± 3 | 11 ± 1 |
| FATP1 (units/mg lipid) | 0.03 ± 0.006 | 0.14 ± 0.05 | 0.04 ± 0.01 | 0.10 ± 0.04 |
| FABP(pm) (units/mg lipid) | 404 ± 161 | 446 ± 102 | 253 ± 89 | 485 ± 103† |
Values represent mean ± SE. SQ, subcutaneous.
†P < 0.05 vs. abdominal SQ within sex group.
Relationships among ACS, DGAT, CD36, FATP1, and FABP(pm).
In omental adipose tissue, all five FA storage pathway factors were highly correlated (r values 0.50–0.98), and the relationships did not appear to differ between sexes. In abdominal subcutaneous adipose tissue, ACS and DGAT activity were well correlated in both women (0.96, P < 0.0001) and men (r = 0.72, P < 0.02). The CD36 content of abdominal subcutaneous adipose tissue also correlated with ACS and DGAT activities in women and men, although the strength of the associations tended to be less strong (r values 0.56–0.83) than we found for omental fat. In women, but not men, ACS activity was correlated with FATP1 (r = 0.76, P < 0.05).
FA storage factors and fat mass.
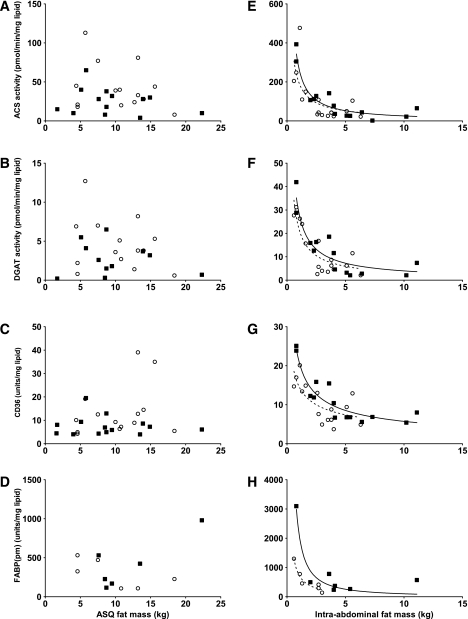
In both men and women, omental ACS activity, DGAT activity, and CD36 and FABP(pm) tissue content per milligram of lipid decreased in a polynomial fashion as a function of visceral fat mass (Fig. 2). FATP1 was not significantly correlated with visceral fat mass (data not shown). Abdominal subcutaneous fat mass was not correlated with ACS or DGAT activity per milligram of adipose tissue lipid or with CD36, FABP(pm), and FATP1 content.
FIG. 2.
Correlation between regional FA storage factors and regional fat mass. Left: Abdominal subcutaneous adipose tissue. A: Relationship between ACS and abdominal subcutaneous adipose tissue fat mass for women r = 0.18, P = 0.65 and for men r = 0.01, P = 0.82. B: Relationship between DGAT and abdominal subcutaneous adipose tissue fat mass for women r = 0.03, P = 0.58 and for men r = 0.07, P = 0.45. C: Relationship between CD36 and abdominal subcutaneous adipose tissue fat mass for women r = 0.12, P = 0.24 and for men r = 0.01, P = 0.78. D: Relationship between plasma membrane FABP(pm) and abdominal subcutaneous adipose tissue fat mass (r and P values for men and women). Right: Omental adipose tissue. E: Relationship between abdominal subcutaneous adipose tissue and omental fat mass for women r = 0.50, P = 0.002 and for men r = 0.88, P < 0.0001. F: Relationship between DGAT and omental fat mass for women r = 0.72, P < 0.0001 and for men r = 0.80, P < 0.0001. G: Relationship between CD36 and omental fat mass for women r = 0.52, P = 0.003 and for men r = 0.85, P < 0.0001. H: Relationship between FABP(pm) and omental fat mass (r and P values for men and women). Solid squares/solid line, men; open circles/dashed line, women; ASQ, abdominal subcutaneous adipose tissue.
Relationships between mRNA levels of adipogenic genes and FA storage factors.
Because of the strong correlations between FA storage proteins and enzymes, we tested whether some early adipogenic upstream regulatory genes could explain these relationships. Genes we examined because of their role in adipogenesis and their synergistic actions included PPAR-γ 1 and 2 and C/EBP-α. The relative levels of gene expression are provided in Table 3. For abdominal subcutaneous adipose tissue, there was a significant, negative correlation between DGAT activity (pmol ⋅ min−1 ⋅ mg lipid−1) and C/EBP-α (r = −0.7, P < 0.05) and PPAR-γ 1 (r = −0.7, P < 0.05) in women. Other abdominal subcutaneous FA storage factors and proteins showed no significant correlation with mRNA levels of C/EBP-α, PPAR-γ 1, or PPAR-γ 2, and for men there were no significant correlations.
TABLE 3.
Adipose tissue gene expression
| Women |
Men |
|||
|---|---|---|---|---|
| Abdominal SQ | Omental | Abdominal SQ | Omental | |
| C/EBP-α | 2.0 ± 0.2† | 1.0 ± 0.2 | 1.5 ± 0.1 | 1.0 ± 0.2 |
| PPAR-γ 1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.3 |
| PPAR-γ 2 | 1.3 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 |
Values (expressed in relative units) represent mean ± SEM. SQ, subcutaneous.
†P = 0.0002 vs. omental adipose tissue within the same sex group.
For women, there was a significant, negative correlation between omental FATP1 (units/mg lipid) and omental PPAR-γ 1 (r = −0.8, P = 0.02) and PPAR-γ 2 (r = −0.7, P = 0.05). Other omental FA storage factors and protein showed no significant correlation with omental mRNA levels of C/EBP-α, PPAR-γ 1, or PPAR-γ 2.
Relationships between direct FFA storage and FA storage factors
Abdominal subcutaneous adipose tissue.
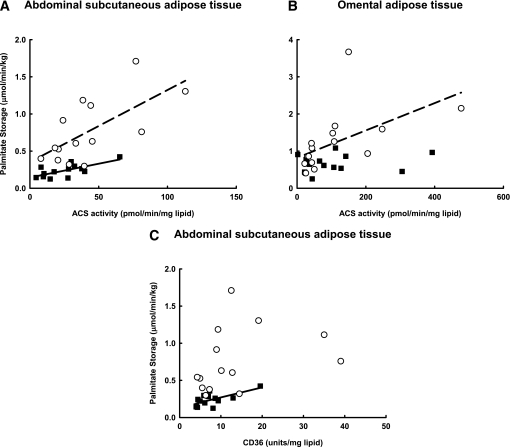
The correlation between adjusted adipocyte lipid SA and abdominal subcutaneous ACS activity was of borderline significance for women (r = 0.49, P = 0.07) and men (r = 0.55, P = 0.05) (Fig. 3). ACS activity was significantly correlated with the calculated direct FFA storage rates in both women (r = 0.66, P = 0.001) and men (r = 0.70, P = 0.007); however, palmitate storage rates were greater in women than men irrespective of ACS activity (Fig. 3), indicating that ACS, and by inference the FA storage factors correlated with ACS, could not account for sex differences in palmitate storage. In men, although the adjusted adipocyte lipid SA did not correlate with CD36 content, the calculated direct FFA storage rates were correlated with CD36 (r = 0.67, P = 0.009). For women, neither variable was correlated with abdominal subcutaneous CD36 content. FFA storage rates were not correlated with FABP(pm) or FATP1 in either sex.
FIG. 3.
Relationships between calculated palmitate storage rates and FA storage factors. A: Relationship between abdominal subcutaneous adipose tissue palmitate storage and ACS for women r = 0.66, P = 0.01 and for men r = 0.70, P = 0.01. B: Relationship between omental palmitate storage and ACS for women r = 0.54, P = 0.03 and for men r = 0.17, P = 0.57. C: Relationship between omental palmitate storage and CD36 for women r = 0.33, P = 0.20 and for men r = 0.67, P = 0.01. Solid squares/solid lines, men; open circles/dashed lines, women.
Omental adipose tissue.
In women, omental ACS activity, which we used as a marker for the other FA storage factors except FATP1 and FABP(pm), was significantly correlated with both adjusted omental adipocyte lipid SA (r = 0.84, P < 0.001) and calculated FFA storage rates (r = 0.54, P = 0.03). Total tissue FATP1 content and plasma membrane FABP(pm) were not correlated with direct FFA storage. In men, neither omental adipocyte lipid SA or calculated direct FFA storage rates were significantly correlated with omental ACS (also the surrogate for CD36 and DGAT), FABP(pm), or FATP1. Multivariate regression analysis indicated that the greater omental palmitate storage rates in women than men were not attributable to differences in ACS activity.
DISCUSSION
The regional differences in direct FFA storage are more akin to sex differences in body fat distribution (14) than are the patterns of meal FA storage (10,11). We conducted these studies to understand whether abdominal subcutaneous and omental adipose tissue direct FFA storage rates are associated with variations in the FA storage properties of adipocytes. We have found that the proportion (14,26) and the rate (27) of FFA stored in subcutaneous adipose tissue via the direct reuptake pathway are greater in women than in men. This is the first description of sex differences in visceral adipose tissue FFA storage and the first examination of the potential role of a panel of FA storage proteins (CD36, FABP[pm], and FATP1) and enzymes (ACS and DGAT) as factors in this process. We found that 1) direct FFA storage rates are significantly greater in omental than abdominal subcutaneous fat in nonobese women and men; 2) the rate of FFA storage is greater in both depots in women than men; 3) both ACS activity and CD36 content per milligram of lipid were correlated with direct FFA storage rates per unit of adipose tissue lipid in abdominal subcutaneous fat in men, whereas only ACS was so correlated in women; 4) the FA storage proteins (except FABP[pm] and FATP1) were correlated with omental direct FFA storage in women, but not in men; and 5) the proteins and enzymes driving FA uptake and storage in visceral fat (per milligram of lipid) are reduced in those with greater amounts of visceral fat.
Few healthy adults undergo intra-abdominal surgery that will allow collection of carefully timed adipose tissue samples. To ensure that our observations regarding direct FFA storage were not affected by acute or chronic illness/inflammation or by medications that might alter FA metabolism, we recruited patients donating a kidney, who must be healthy to be accepted as a donor, and patients undergoing elective cholecystectomy with no other acute or chronic medical problems. The consistency of our results suggests that we accomplished our goal of understanding the processes regulating direct FFA storage in visceral and subcutaneous adipose tissue in normal adults. Because the unique regulation of different adipose tissue depots in humans is not readily recapitulated in rodent models, our findings are especially relevant to human health and disease.
We previously reported that women with increased visceral fat mass had reduced dietary FA (28) and direct FFA storage (15) per unit of fat mass, but did not have an explanation for the observation. We report that the amount of CD36 and FABP(pm), and ACS and DGAT activity per milligram of lipid were substantially less in omental fat from men and women with greater visceral fat mass. This is in contrast with abdominal subcutaneous fat, where these FA storage factors were not different in those with lesser or greater fat mass. Combined, these observations suggest that accumulation of excess visceral fat is not due to an enhanced ability to store FAs compared with other depots, but occurs in the context of reduced FA storage machinery. A parallel reduction in rates of lipolysis per kilogram of omental fat would seem to be required for visceral fat mass to remain stable. As a contributor to total fat mass, direct FFA storage is quantitatively less important than LPL-mediated storage of dietary FAs. Indeed, during the postprandial period, LPL-dependent FA storage is ∼4.5 times greater than LPL-independent storage (29). Our hypothesis is that the direct FFA storage pathway plays a role in the redistribution of fat between depots and over more extended periods of time, separate from the large inflows of FAs to adipose tissue that can occur over short periods of time after meal ingestion (30).
The plasma FFA concentrations we found in these patients were ∼50% greater than we see in the more relaxed setting of a General Clinical Research Center, where we also have ideal control over the timing of the experiment. Patients undergoing even elective surgery may be nervous/stressed and subject to somewhat longer periods of fasting, both of which will increase FFA concentrations (31,32). However, given that FFA concentrations can range from <10 μmol/L (33) to >2,000 μmol/L (34), the average concentrations of ∼540 (palmitate concentrations of 135) μmol/L we observed in this population are close to the typical overnight postabsorptive values of 400–500 μmol/L. Nevertheless, this may not be completely representative of the typical postabsorptive state, where lipolysis is ∼20% less and one can expect a smaller extracellular to intracellular gradient against which FFA must compete to be incorporated in TAG.
Counter to our hypothesis, the omental adipose tissue FFA storage rates were greater in women than men, although men had ∼50% more visceral fat. However, the percent of FFA stored in visceral fat was similar in both groups. We postulate that women compensate for the greater direct FFA storage rates by either increased release or reduced storage of meal FAs compared with men. Whereas we have not observed a trend for reduced meal FA storage in men compared with women (9), we did notice that for any given amount of visceral fat, the proportion of hepatic FFA delivery from visceral fat was greater in women than men (6).
The storage rates of FFA in omental fat correlated with some of our markers of FA storage activity, which were in turn highly correlated with each other. This suggests that in visceral adipose tissue there is a coordinated regulation of some adipocyte factors that promote FA storage. In contrast, abdominal subcutaneous adipose tissue CD36 was not as tightly correlated with ACS and DGAT, especially in men. This allowed us to test whether the amount of CD36 was independently associated with FFA storage rates. In both men and women, ACS (also serving as a surrogate for DGAT) was correlated with FFA storage. In men, but not women, there were significant, independent associations of FFA storage to ACS and CD36. These findings suggest that intracellular processing steps, which tend to be coregulated in both omental and subcutaneous adipose tissue, help determine direct FFA storage. In men, membrane transport factors (CD36) also seem to be a rate-limiting step. FABP(pm) is a protein that facilitates FA transport into 3T3-L1 cells via a saturable process. In humans, FABP(pm) is known to be present in skeletal muscle (21), playing a role in FFA uptake, and is upregulated with endurance training (35). The presence and characterization of FABP(pm) in human adipocyte plasma membranes have not been previously described. Although we did not detect a significant correlation between adipocyte plasma membrane FABP(pm) content and direct FFA storage, additional studies will be needed to fully characterize the contribution of FATP1 and FABP(pm) to FFA storage in human adipose tissue.
Because of the striking correlation among CD36, ACS, and DGAT, especially in omental fat, the next logical step would be to look for upstream regulatory genes that orchestrate the FA storage process. The early, adipogenic regulatory genes, C/EBP-α and PPAR-γ, seemed logical candidates because both have been reported to regulate FA storage factors. We did not find significant correlations between most of the FA storage factors we measured and omental C/EBP-α and PPAR-γ 1 or 2. Of note, C/EBP-α and the PPARs showed a negative correlation with several esterification pathway factors. This could suggest a protective “feedback” mechanism to counter excess adipose tissue lipid accumulation.
There are several limitations to this study. We were unable to measure FFA turnover in the surgical suite because that would have entailed another level of complexity given the nature of the surgical procedures. Thus, our FFA flux estimates are based on a regression formula that can predict 65% of the variance in this value. Had we been able to measure FFA flux directly, we may have found stronger associations between adipocyte factors and FFA storage rates. As mentioned, the plasma FFA concentrations were slighter greater than we typically observe, possibly because of preoperative stress, which could somewhat alter FFA storage relative to the usual overnight postabsorptive state. Our population of patients was generally healthy, and thus the results cannot be extrapolated to those with diabetes, polycystic ovarian disease, and other conditions associated with more pathologic visceral fat accumulation.
In summary, we report the first assessment of direct FFA storage in visceral and abdominal subcutaneous fat in men and women. In addition, we have measured a number of the proteins involved in FA cell membrane transport and enzyme activities for esterification/trapping (ACS) and final storage of FAs as triglyceride (DGAT). In light of these data, direct FFA storage in the postabsorptive state does not seem to play a role in the sex-specific differences in visceral adipose tissue accumulation. In addition, none of the five FA storage factors we measured could statistically account for the greater direct FFA storage rates in women than men in either adipose tissue depot. Our findings do suggest that visceral fat downregulates most or all of the steps in FA storage as fat mass increases. Despite this, omental fat stores FFA at rates two to three times greater than abdominal subcutaneous fat in nonobese adults, likely because of greater amounts of FA storage proteins. We believe these results shed new light on the factors that determine FA storage in adipose tissue and highlight some of the sex differences in this process that will help explain differences in body fat distribution.
ACKNOWLEDGMENTS
This study was funded by National Institutes of Health grants DK-40484, DK-45343, and DK-50456; Grant 1 UL1 RR024150 from the National Center for Research Resources; the Women in Science and Engineering Program and Integrative and Evolutionary Biology Program, the University of Southern California; and the Mayo Foundation. Financial support was provided for clinical research by Stockholm County Council and the Erling-Persson Family Foundation.
No potential conflicts of interest relevant to this article were reported.
A.H.A. researched data, contributed to discussion, wrote the manuscript, reviewed and edited the manuscript, and performed studies. C.K. and M.M. researched data, contributed to discussion, reviewed and edited the manuscript, and performed studies. M.D.S., J.K.H., and S.J.T. contributed to discussion, reviewed and edited the manuscript, and assisted in performance of studies. J.N. and A.T. researched data, contributed to discussion, reviewed and edited the manuscript, and assisted in performance of studies. L.D.B. researched data and performed laboratory work. L.P.T. researched data, reviewed and edited the manuscript, and performed laboratory work. D.B. researched data, contributed to discussion, reviewed and edited the manuscript, and performed laboratory work. M.D.J. oversaw the study, performed studies, contributed to discussion, and wrote the manuscript.
The authors thank Christy C. Allred, Deborah Harteneck, and Lendia Zhou for performing laboratory work; Carley Vrieze for coordinating the studies; Barb Norby for performing studies (all from the Endocrine Research Unit, Mayo Clinic, Rochester, MN); and the Mayo Clinic Clinical Research Unit for providing continuous support.
REFERENCES
- 1.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev 1994;74:761–811 [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48 [DOI] [PubMed] [Google Scholar]
- 3.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959 [DOI] [PubMed] [Google Scholar]
- 4.Guo ZK, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 1999;48:1586–1592 [DOI] [PubMed] [Google Scholar]
- 5.Burguera B, Proctor DN, Dietz N, Guo Z, Joyner MJ, Jensen MD. Leg free fatty acid kinetics during exercise in men and women. Am J Physiol Endocrinol Metab 2000;278:E113–E117 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mårin P, Rebuffé-Scrive M, Björntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 1990;20:158–165 [DOI] [PubMed] [Google Scholar]
- 8.Mårin P, Lönn L, Andersson B, et al. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 1996;81:1018–1022 [DOI] [PubMed] [Google Scholar]
- 9.Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA. Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab 2003;285:E1282–E1288 [DOI] [PubMed] [Google Scholar]
- 10.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 2000;279:E455–E462 [DOI] [PubMed] [Google Scholar]
- 11.Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol Endocrinol Metab 2005;288:E547–E555 [DOI] [PubMed] [Google Scholar]
- 12.Oakes ND, Kjellstedt A, Forsberg GB, et al. Development and initial evaluation of a novel method for assessing tissue-specific plasma free fatty acid utilization in vivo using (R)-2-bromopalmitate tracer. J Lipid Res 1999;40:1155–1169 [PubMed] [Google Scholar]
- 13.Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol 1999;276:E233–E240 [DOI] [PubMed] [Google Scholar]
- 14.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 2007;56:1369–1375 [DOI] [PubMed] [Google Scholar]
- 15.Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD. Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes 2008;57:1186–1194 [DOI] [PubMed] [Google Scholar]
- 16.Hannukainen JC, Kalliokoski KK, Borra RJ, et al. Higher free fatty acid uptake in visceral than in abdominal subcutaneous fat tissue in men. Obesity (Silver Spring) 2010;18:261–265 [DOI] [PubMed] [Google Scholar]
- 17.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995;61:274–278 [DOI] [PubMed] [Google Scholar]
- 18.Miles JM, Ellman MG, McClean KL, Jensen MD. Validation of a new method for determination of free fatty acid turnover. Am J Physiol 1987;252:E431–E438 [DOI] [PubMed] [Google Scholar]
- 19.Allred CC, Krennmayr T, Koutsari C, Zhou L, Ali AH, Jensen MD. A novel ELISA for measuring CD36 protein in human adipose tissue. J Lipid Res 2011;52:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatch GM, Smith AJ, Xu FY, Hall AM, Bernlohr DA. FATP1 channels exogenous FA into 1,2,3-triacyl-sn-glycerol and down-regulates sphingomyelin and cholesterol metabolism in growing 293 cells. J Lipid Res 2002;43:1380–1389 [DOI] [PubMed] [Google Scholar]
- 21.Turcotte LP, Swenberger JR, Tucker MZ, et al. Muscle palmitate uptake and binding are saturable and inhibited by antibodies to FABP(PM). Mol Cell Biochem 2000;210:53–63 [DOI] [PubMed] [Google Scholar]
- 22.Hall AM, Smith AJ, Bernlohr DA. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem 2003;278:43008–43013 [DOI] [PubMed] [Google Scholar]
- 23.Coleman RA. Diacylglycerol acyltransferase and monoacylglycerol acyltransferase from liver and intestine. Methods Enzymol 1992;209:98–104 [DOI] [PubMed] [Google Scholar]
- 24.Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity (Silver Spring) 2009;17:1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sewter C, Blows F, Considine R, Vidal-Puig A, O’Rahilly S. Differential effects of adiposity on peroxisomal proliferator-activated receptor gamma1 and gamma2 messenger ribonucleic acid expression in human adipocytes. J Clin Endocrinol Metab 2002;87:4203–4207 [DOI] [PubMed] [Google Scholar]
- 26.Koutsari C, Snozek CL, Jensen MD. Plasma NEFA storage in adipose tissue in the postprandial state: sex-related and regional differences. Diabetologia 2008;51:2041–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 2011;60:2032–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD. Meal fatty acid uptake in visceral fat in women. Diabetes 2007;56:2589–2597 [DOI] [PubMed] [Google Scholar]
- 29.Bickerton AS, Roberts R, Fielding BA, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 2007;56:168–176 [DOI] [PubMed] [Google Scholar]
- 30.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 2006;291:E1115–E1123 [DOI] [PubMed] [Google Scholar]
- 31.Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 2009;587:5939–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottschalk LA, Stone WM, Gleser GC, Iacono JM. Anxiety and plasma free fatty acids (FFA). Life Sci 1969;8:61–68 [DOI] [PubMed] [Google Scholar]
- 33.Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 1989;38:1595–1601 [DOI] [PubMed] [Google Scholar]
- 34.Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest 1987;79:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiens B, Kristiansen S, Jensen P, Richter EA, Turcotte LP. Membrane associated fatty acid binding protein (FABPpm) in human skeletal muscle is increased by endurance training. Biochem Biophys Res Commun 1997;231:463–465 [DOI] [PubMed] [Google Scholar]