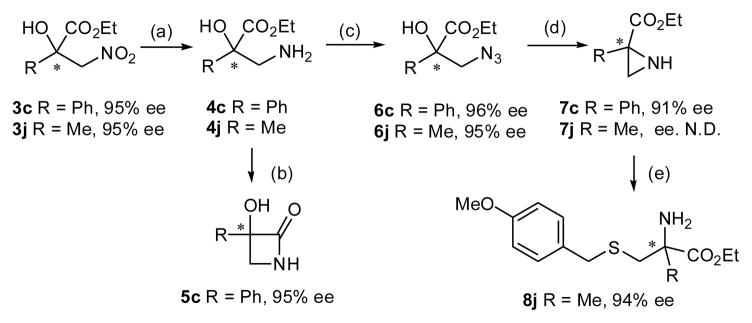
Scheme 2.
Asymmetric Synthesis of β-Lactam, Aziridine and α-Methylcysteine Derivatives a
(a) Raney Ni, H2 (1atm); (b) i-PrMgCl, 38% y. over 2 steps; (c) TfN3, CuSO4(cat.), for 6c, 84 % y. over 2 steps; for 6j, 63% y. over 2 steps; (d) PPh3, CH3CN, for 7c, 80% y.; for 7j, 71% y.; (e) BF3. Et2O, p-Methoxybenzyl mercaptan, 56% y.
aSee Supporting Information for details.