Table 1.
Enantioselective Nitroaldol Addition of Nitromethane to α-Ketoester 2a.a
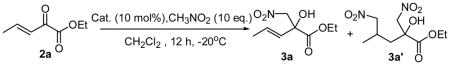 | ||||
|---|---|---|---|---|
| Entry | Cat.b | Conv./% c | 3a/3a′c | ee/% d |
| 1 | Et3N | >95 | 80/20 | -- |
| 2 | QD | 91 | >95/5 | −17 |
| 3 | DHQD-PHN | 74 | >95/5 | 59 |
| 4 | (DHQD)2AQN | >95 | >95/5 | 40 |
| 5 | β-ICD | >95 | >95/5 | 61 |
| 6 | QD-1a | >95 | >95/5 | 86 |
| 7 | QD-1b | 93 | >95/5 | 70 |
| 8 | QD-1c | 93 | >95/5 | 93 |
| 9 | QD-1d | >95 | >95/5 | 97 |
| 10 | Q-1d | >95 | >95/5 | −97 |
Unless noted, reactions were carried out with 0.1 mmol of 2a, 1 mmol CH3NO2 in 0.1 mL CH2Cl2 with 10 mol% catalyst at −20°C for 12h.
See Supporting Information for the structure of the catalysts.
Determined by 1H NMR analysis.
Determined by HPLC analysis
