Table 2.
Enantioselective Nitroaldol Addition of Nitromethane to α-Ketoester 2 Catalyzed by QD-1d and Q-1d (in brackets)a
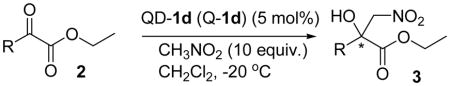 | |||||
|---|---|---|---|---|---|
| Entry | R | Time/h | yield/% b | ee/% c | |
| 1 | 2a |
|
14 (15) | 92 (92) | 96 (97) |
| 2 | 2b |
|
24 (24) | 98 (99) | 94 (95) |
| 3 | 2c | Ph- | 35 (46) | 96 (96) | 95d (93) |
| 4 | 2d | 4-MeO-Ph- | 96 (96) | 86 (84) | 94 (97) |
| 5 | 2e | 4-MeS-Ph- | 72 (72) | 86 (86) | 96 (96) |
| 6 | 2f | 4-Cl-Ph- | 12 (12) | 98 (96) | 97d (96) |
| 7 | 2g | 4-CN-Ph- | 9 (11) | 96 (98) | 94 (97) |
| 8 | 2h | 3-Cl-Ph- | 11 (11) | 91 (96) | 95 (95) |
| 9 | 2i | 2-Naphthyl- | 60 (60) | 96 (97) | 94 (94) |
| 10 | 2j | Me- | 12 (12) | 89 (90) | 95 (95) |
| 11 | 2k | n-Pr- | 17 (15) | 90 (90) | 93 (93) |
| 12 | 2l |
|
14 (11) | 88 (89) | 95 (94) |
| 13 | 2m |
|
15 (11) | 87 (86) | 94 (93) |
Unless noted, reactions were run with 0.5 mmol of 2, 5 mmol CH3NO2 in 0.5 mLCH2Cl2 with 5 mol% QD-1d, the results in parentheses were obtained with Q-1d to give opposite enantiomer, see Supporting Information for details.
Isolated yield.
Determined by HPLC analysis.
The absolute configuration is determined to be S, see Supporting Information for details.
