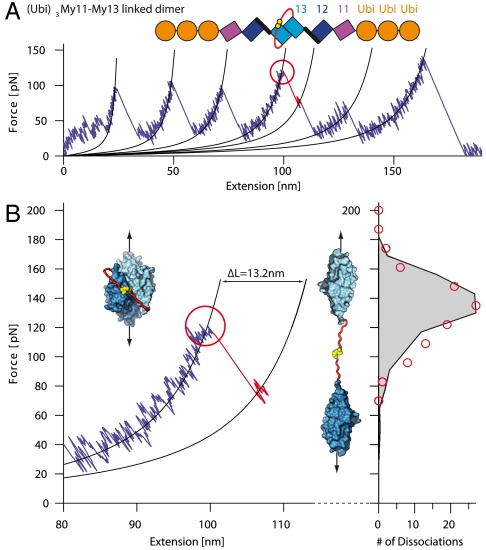
Fig. 4.
Forced dissociation of linked My13 dimer domains. (A) Typical force-extension trace of (Ubi)3My11-My13 linked dimers. A schematic representation of the construct is given on top. Each monomer consists of three ubiquitins (orange) and the myomesin domains My11-My13 (purple, blue, and cyan). The two monomers are linked via an unstructured amino acid sequence (red) with C-terminal cysteines (yellow) that form a covalent disulfide bond. Dimer dissociation (red circle) is identified by a shorter contour length increase than for domain unfolding. (B) Zoom into part of (A) where dimer dissociation occurs. Insets show structure of the My13 domains (cyan) with linker (red) and bonded cysteines (yellow) in the dimeric (Left) and dissociated state (Right). The dissociation force distribution to the right (red circles) is well reproduced by a Monte Carlo simulation (black trace).