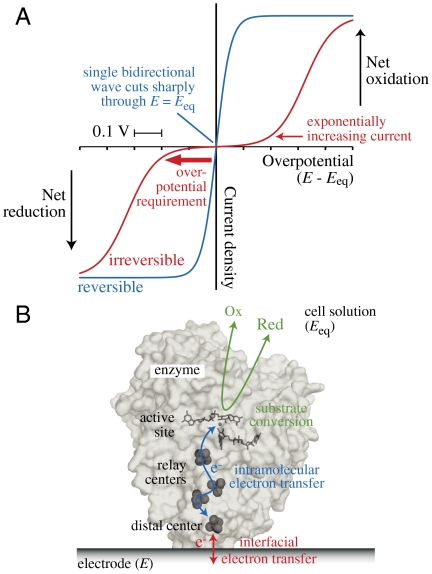
Fig. 1.
Concepts applied in this article. (A) Steady-state electrochemical kinetics visualized by cyclic voltammetry. When both the oxidized and reduced forms of a redox-active species are present, a reversible electrochemical reaction (one with a large exchange current density) produces a single sigmoidal wave (blue) that cuts (without inflection) through the zero-current axis at the equilibrium potential (Eeq) and achieves a potential-independent limiting current in either direction at relatively low overpotential. Conversely, if the exchange current density is low, the current is negligible around Eeq and two sigmoidal waves (red), one for either direction, are separated in potential, emerging from the baseline with an exponential dependence on potential: A substantial overpotential is required to match the current produced by the reversible system. (B) Cartoon showing an adsorbed enzyme functioning as a molecular electrocatalyst.