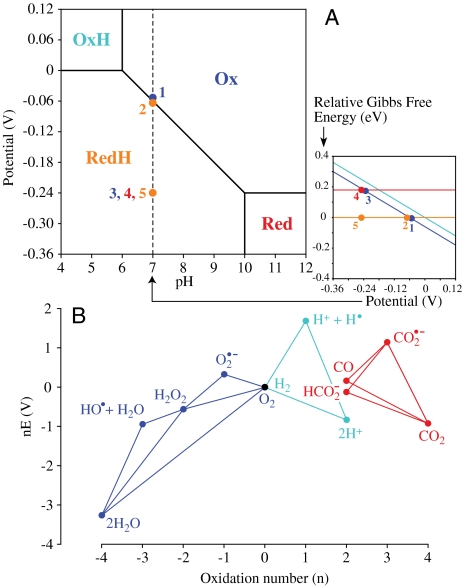
Fig. 3.
Overpotential requirements resulting from high-energy catalytic intermediates. (A) The Pourbaix diagram for a PCET reaction denotes the most stable species at each potential and pH. PCET reactions occur at intermediate pH, when only the reduced species (Red) is protonated. The inset shows a vertical slice through the main diagram at pH 7, showing how the energy of each state varies, relative to RedH. Concerted PCET reaction, 1 → 2 (no overpotential); stepwise reaction, 1 → 3 (overpotential), 3 → 4 (electron transfer), 4 → 5 (spontaneous H+ transfer). See text for details. (B) Frost (oxidation state) diagram showing the redox reactions of H+/H2, O2/H2O (49), and CO2/CO or formate (pH 7) (16, 17). The slope of the line connecting two species gives the reduction potential for their interconversion; intermediates above connecting lines are unstable with respect to disproportionation. The vertical displacement of an intermediate, above the line for the substrate reaction, indicates the overpotential requirement associated with its formation.