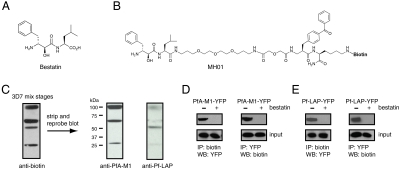
Fig. 1.
Identification of PfA-M1 and Pf-LAP as the targets of the antiparasitic MAP inhibitor bestatin. (A) Structure of bestatin. (B) Structure of MH01. (C) Identification of PfA-M1 and Pf-LAP as the parasite targets of bestatin. Parasite lysates were prepared by freeze-thaw lysis and subsequently labeled with 5 μM MH01, UV crosslinked, and analyzed by Western blot for biotin. Four proteins were labeled by MH01. The same blot was stripped and reprobed sequentially with antibodies for PfA-M1 and Pf-LAP; three MH01 labeled proteins were accounted for by PfA-M1 and fourth by Pf-LAP. (D) A parasite line expressing a YFP-tagged PfA-M1 protein was incubated with MH01 followed by immunoprecipitation for biotin (MH01) and Western blot analysis for YFP, which confirmed that PfA-M1 is targeted by MH01 (first panel). The reciprocal experiment involving the immunoprecipitation of PfA-M1-YFP (after incubation of parasites with MH01) using a YFP-specific antibody confirmed PfA-M1-YFP is biotinylated and thus labeled by MH01 (second panel). (E) Likewise, the same analysis was performed using a parasite line expressing a YFP-tagged Pf-LAP protein; incubation of these parasites with MH01, followed by immunoprecipitation of biotin (MH01) and Western blot analysis for YFP confirmed that MH01 labels Pf-LAP. The reciprocal experiment involving immunoprecipitation of Pf-LAP-YFP and analysis by Western blot for biotin confirmed that Pf-LAP-YFP is biotinylated by MH01. Lastly, MH01 labeling of YFP-tagged MAPs, in both cases, is blocked by pretreatment with unbiotinylated bestatin as seen as a lack of labeling. Input lanes below each panel show Western blot analysis using anti-YFP of total parasite lysate just before immunoprecipitation.