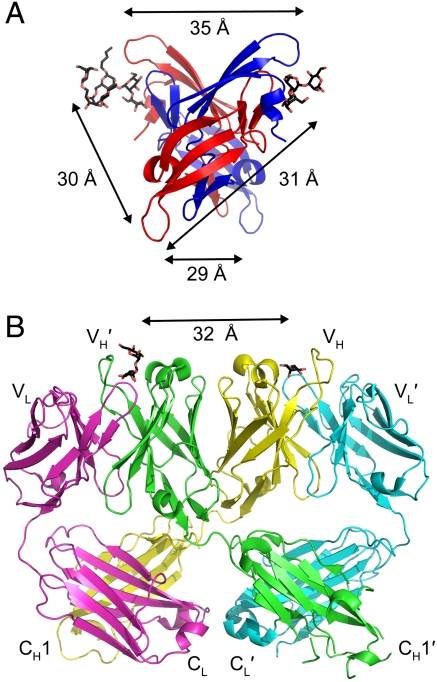
Fig. 5.
Similarity in carbohydrate binding site spacing in CV-N and the 2G12 anti-HIV (Fab)2. (A) Each of the four carbohydrate binding sites in one WT CV-N crystal structure (15) (P41212 space group) is approximately 30 to 35 Å from the other sites (structure is viewed from the bottom with respect to Fig. 1). Carbohydrates (shown as sticks with black carbons and red oxygens) were only resolved in the A binding sites in the crystal structure. (B) Ribbon diagram of the domain-swapped (Fab)2 from IgG 2G12, a broadly neutralizing antibody specific for carbohydrates on gp120 (35). The domain swapping creates a rigid (Fab)2 dimer in which the carbohydrate binding sites at the antigen combining sites are spaced approximately 30 to 35 Å apart. Carbohydrates are shown as sticks with black carbons and red oxygens and antibody domains are labeled.