Abstract
Background
Excessive corticosteroid exposure is associated with atrophic effects on the human hippocampus and amygdala. These effects appear to be, at least in part, mediated through corticosteroid-induced release of glutamate. We previously reported that lamotrigine, a glutamate-release inhibitor, significantly improved declarative memory but did not change hippocampal volume, as compared to placebo, in corticosteroid-treated patients. To our knowledge, no data are available on preventing or reversing the impact of corticosteroids on the amygdala.
Methods
We examined the effects of 24 weeks of randomized, placebo-controlled lamotrigine therapy on amygdala volume and mood in 28 corticosteroid-treated patients (n = 12 for placebo, n = 16 for lamotrigine). Amygdala volumes were measured from tracings of the MR images from weeks 0 and 24. Mood was assessed every two weeks with Hamilton Depression Rating Scale (HAM-D) and Young Mania Rating Scale (YMRS).
Results
An ANCOVA revealed that patients on lamotrigine had significantly larger left amygdala volume at week 24 than patients on placebo after controlling for baseline volume. Neither exit nor week 24 ANCOVAs of HAM-D and YMRS revealed significant difference between lamotrigine and placebo groups.
Conclusions
Results suggest that lamotrigine attenuated the effects of corticosteroids on the left amygdala. Larger trials are warranted to confirm these findings.
Keywords: amygdala, corticosteroid, lamotrigine, volume, atrophy
INTRODUCTION
Excess corticosteroid exposure in animals and humans is associated a variety of central nervous system effects. A variety of medications including, but not limited to, tamoxifen1, retinoic acid2, interferon-alfa3 and antihypertensives4, are associated with mood changes. In humans, corticosteroids are associated with mood symptoms including mania, depression and even psychosis that have been reported in the literature since the first use of these medication approximately 60 years ago.5, 6 Cognitive effects, particularly deficits in declarative memory, are also common during corticosteroid exposure.5, 7, 8 Consistent with change in declarative memory, corticosteroid excess is associated with hippocampal atrophy.7, 9
Another brain region sensitive to the effects of corticosteroids is the amygdala. Animal studies on stress-induced release of corticosteroids have demonstrated enhanced dendritic arborization in the basolateral complex of the amygdala (BLA) during stress immobilization and, in contrast, atrophy of these neurons during chronic unpredictable stress.10 Acute and chronic administration of exogenous corticosteroids to rats also resulted in dendritic hypertrophy in the BLA.11
Human studies indicate children with classic congenital adrenal hyperplasia12 and Cushing’s disease13 present with decreased amygdala volumes compared to controls. Moreover, adults with asthma or rheumatic diseases on long-term corticosteroid therapy had 20% smaller left and 11% smaller right amygdala volumes than controls.14 Amygdala atrophy is also found in a variety of psychiatric disorders, including posttraumatic stress disorder15, unmedicated depression16 and bipolar disorder.17
Animal studies demonstrate that hippocampal changes due to corticosteroids can be prevented with N-methyl-D-aspartate (NMDA) antagonists18 and agents, such as phenytoin, that decrease glutamate release.19 In humans, controlled trials suggest that mood symptoms with corticosteroids may be prevented with phenytoin20 or lithium21, while declarative memory appears to improve using the NMDA antagonist memantine22 and the glutamate release inhibitor lamotrigine.23
In this report, we examined mood and amygdala volume in corticosteroid-treated patients given up to 24 weeks of lamotrigine or placebo. Given our prior report of amygdala atrophy during prescription corticosteroid therapy14 and the mood-stabilizing properties of lamotrigine in bipolar disorder24, we hypothesized differences in mood and amygdala volume with lamotrigine as compared to placebo.
MATERIALS AND METHODS
For a randomized, double-blinded study, twenty-eight patients receiving chronic oral corticosteroid therapy were recruited from UT Southwestern Medical Center clinics from August 2002 to March 2005 by trained research assistants. Upon signing an IRB-approved consent form, participants were assessed for mood with the Hamilton Depression Rating Scale (HAM-D) and Young Mania Rating Scale (YMRS) at baseline and every two weeks thereafter at the UT Southwestern Psychoneuroendocrine Research Program clinic for up to 24 weeks. Brain magnetic resonance imaging on a 1.5 Tesla scanner was obtained at baseline and week 24. Participants of ages 18–65 years receiving at least 7.5 mg of prednisone for at least six months with no mental illnesses other than those induced by corticosteroids were included in the study. Assessments and exclusionary criteria are further elaborated in our previous paper examining the effects of lamotrigine on memory and hippocampal volume.23 The study was registered at Clinicaltrials.gov (NCT00223262).
A staff member without patient contact randomly allocated participants to a treatment group using randomizer.org and assigned numbered containers to a blinded staff member who administered the medication. Lamotrigine or identical placebo was titrated to 400 mg/day over 10 weeks unless side effects required a slower titration or dose reduction. Treatment group assignment remained anonymous to the researchers until all data collection was complete.
Two reliable, blind raters (SD, SK) conducted anatomical measurements using BRAINS2 software25 under the supervision of MUS. Amygdala volumes were manually traced using methods delineated by Keshavan et al.26 Raters had an intra-class correlation of 0.96 for the right amygdala and 0.95 for the left amygdala as measured on 10 scans. All measurements were adjusted for total brain volume (TBV), which was measured in our previous study on hippocampal volume changes by raters who had an intra-class correlation of 0.95 as measured on 10 scans.23
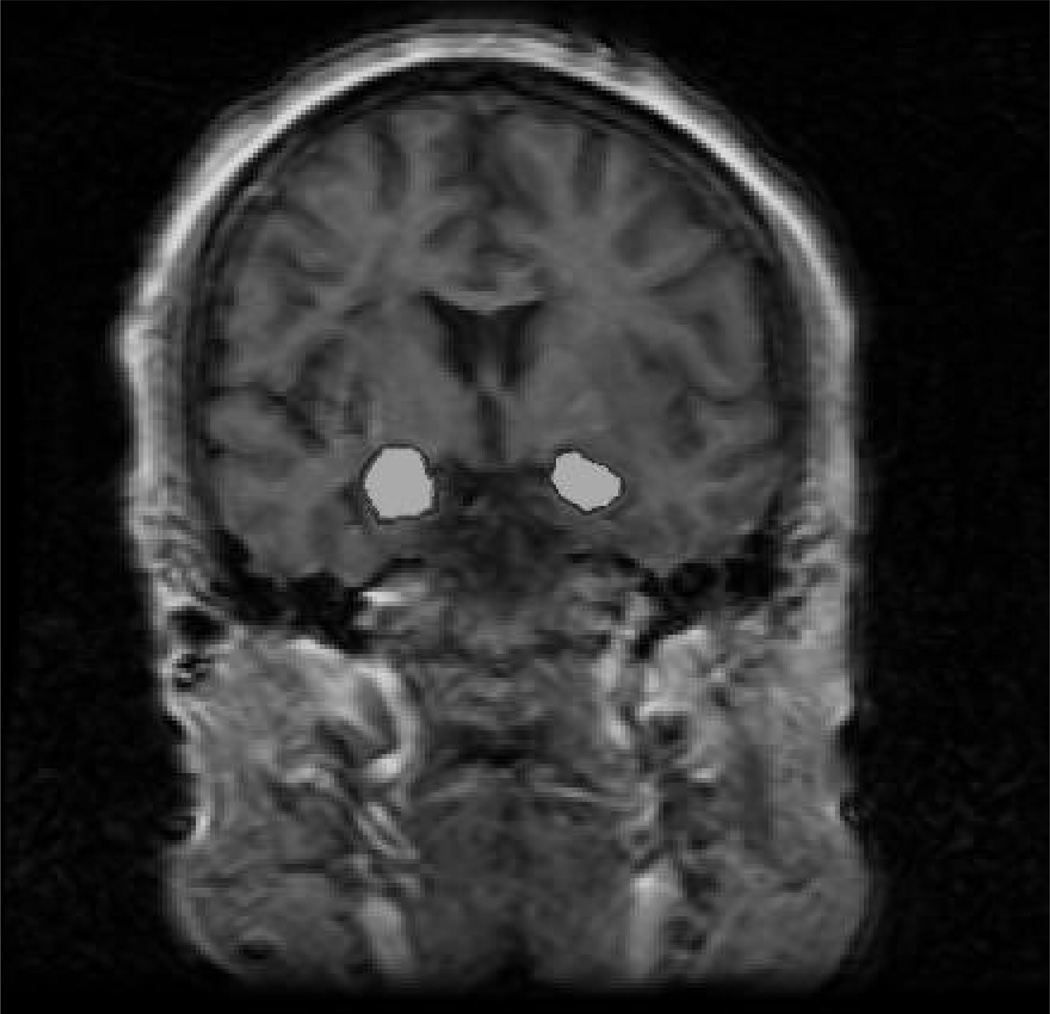
Amygdala is depicted in Figure 1 and defined as follows. The anterior tracings began where temporal and frontal lobes fused and the temporal stem became visible. The anterior-most point of the mamillary body demarcated the amygdala from hippocampus posteriorly. Alveus of the hippocampus formed the inferior boundary, while the temporal stem created the lateral and superior boundaries.26 Cisternal cerebral fluid formed amygdala’s medial boundary.17
Figure 1.
MRI slice from a study participant obtained anterior to the mamillary bodies with right and left amygdala shaded.
To test the effects of lamotrigine on amygdala volume (primary aim), a univariate analysis of covariance (ANCOVA) was performed for exit (week 24) amygdala volume/TBV with covariates of baseline amygdala volume/TBV, corticosteroid dose and duration, and fixed factors of gender and treatment group. Covariates and fixed factors were selected that showed significant between-group differences at baseline, correlated with the outcome measure, or that, based on prior research, could have an impact on the outcome. Analogous ANCOVAs were conducted for assessing mood (secondary aim) with exit HAM-D and YMRS scores, using baseline mood scores, corticosteroid dose and duration as covariates and gender and treatment group as fixed factors. Baseline amygdala/TBV was correlated with baseline mood scores, TBV, current prednisone dose, and duration of corticosteroid therapy using Pearson's correlation coefficient. Sample size was based on our previous lamotrigine study that studied cognitive changes using Rey Auditory Visual Learning Test (RAVLT) scores.23
RESULTS
The 28 participants were randomized (n = 16 lamotrigine and n = 12 placebo), with 27 patients (n = 15 lamotrigine and n = 12 placebo) returning for at least one post-baseline assessment (intent-to-treat sample) and 22 (n = 14 lamotrigine and n = 8 placebo) completing the baseline MRI. Eighteen participants (n = 8 lamotrigine and n = 10 placebo) completed the mood assessments through week 24, and 14 participants (n = 7 lamotrigine and n = 7 placebo) attended both baseline and week 24 MRI scans. Concomitant medications and reasons for study discontinuation, including adverse events, of subjects are discussed in our previous paper.23 Baseline characteristics except prednisone dose did not differ significantly between lamotrigine and placebo groups in the intent-to-treat sample (Table 1).
Table 1.
Demographic Characteristics at Baseline for the Intent-to-treat Sample
| Treatment Group | Lamotrigine (N=15) |
Placebo (N=12) |
p-value |
|---|---|---|---|
| Mean age (SE, range) | 45.6 (2.3, 25–62) | 47.5 (3.7, 24–64) | 0.653 |
| Males, N (%) | 8 (53.3) | 9 (75) | 0.264 |
| Education, N (%) | 0.520 | ||
| High school or less | 7 (46.6) | 4 (33.3) | |
| Some college or more | 8 (53.3) | 8 (66.6) | |
| Ethnicity, N (%) | 0.382 | ||
| Caucasian | 7 (46.6) | 5 (41.6) | |
| African-American | 6 (40.0) | 4 (33.3) | |
| Hispanic | 2 (13.3) | 2 (13.3) | |
| Other | 0 | 1 (6.6) | |
| Concomitant medications | 0.734 | ||
| Number of medications/patient (SE) | 5.2 (0.6) | 5.1 (0.8) | |
| Medical condition requiring prednisone, N (%) | 0.416 | ||
| Renal transplant | 12 (80) | 11 (91.6) | |
| Rheumatic disease | 3 (20) | 1 (8.3) | |
| Current prednisone dose in mg/day (SE) | 18.7 (3.3) | 8.8 (0.6) | 0.038 |
| Prednisone therapy duration in months (SE) | 84.1 (25.4) | 42.8 (14.9) | 0.184 |
Abbreviations: SE, standard error; TBV, total brain volume
The left exit amygdala/TBV ANCOVA revealed significant effects of treatment group [F(1,14) = 6.036, p = 0.044] whereas for the right exit amygdala volume, treatment group [F(1,14)= 2.676, p = 0.146] did not attain significance (Table 2). Neither exit [F(1,27) = 0.804, p = 0.381] nor week 24 [F(1,18) = 1.939, p = 0.191] HAM-D ANCOVAs showed a significant treatment group effect (Table 2). Similarly, between-group differences did not reach significance in either exit [F(1,27) = 0.573, p = 0.458] or week 24 [F(1,18) = 2.674, p = 0.130] YMRS ANCOVAs (Table 2)
Table 2.
Right and Left Adjusted Amygdala Volumes (cm3/cm3) and Mood Scores from Baseline to Exit in Corticosteroid-treated Patients Given Lamotrigine or Placebo
| Baseline | Exit | Change from Baseline to Exit |
||||
|---|---|---|---|---|---|---|
| Lamotrigine Mean (SE) |
Placebo Mean (SE) |
Lamotrigine Mean (SE) |
Placebo Mean (SE) |
Lamotrigine Mean (SE) |
Placebo Mean (SE) |
|
| Right Amygdala/TBV × 100 | 0.156 (0.008) | 0.174 (0.006) | 0.152 (0.008) | 0.160 (0.006) | −0.004 (0.007) | −0.014 (0.005) |
| Left Amygdala/TBV × 100 | 0.152 (0.010) | 0.161 (0.007) | 0.147 (0.009) | 0.153 (0.010) | −0.005 (0.008) | −0.008 (0.007) |
| HAM-D Score | 14.6 (3.0) | 8.5 (2.3) | 13.1 (2.7) | 5.0 (1.6) | −1.5 (2.1) | −3.5 (1.5) |
| YMRS Score | 5.6 (1.1) | 3.6 (1.0) | 4.4 (0.8) | 2.4 (0.9) | −1.2 (1.0) | −1.2 (0.6) |
Abbreviations: SE, standard error; HAM-D, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; TBV, total brain volume
Trends toward significant negative baseline correlations were observed between current prednisone dose and left baseline amygdala volume (r = -0.381, p = 0.081) and between baseline YMRS scores and right baseline amygdala volume (r = −0.364, p = 0.096).
DISCUSSION
We previously reported reduction in both the hippocampus7 and amygdala14 in corticosteroid-treated patients. Animal literature however suggests glutamate-release inhibitors prevent the effects of corticosteroid on the hippocampus.18, 19 In humans, the glutamate-release inhibitor lamotrigine improved declarative memory but did not ameliorate the atrophic effects of corticosteroids on the hippocampus.23 To our knowledge, the impact of glutamate-release inhibitors on the amygdala had not been explored until the current study.
The principal finding of this study is that lamotrigine attenuated the reduction of amygdala volume in corticosteroid-treated patients. Thus, lamotrigine may be associated with improvement in memory and morphological brain changes in corticosteroid-treated patients.
We found a negative trend between baseline left amygdala volume and current prednisone dose, corresponding with our prior report that found an inverse relationship between hippocampal volume and prednisone dose in these same patients.7 However, in another report, we observed a negative correlation between amygdala volume and prednisone duration but not dose.14 Thus, the interaction between prednisone exposure and amygdala volume seems complex. We found another negative trend between baseline YMRS score and right baseline amygdala volume, implying that right amygdala volume may be inversely associated with severity of manic symptoms in corticosteroid-treated patients.
When assessing the findings of this study, several limitations must be considered. First, our sample size was modest and larger trials are warranted to confirm these preliminary observations. Second, our sample population was complex; the patients had serious medical illnesses and were on multiple concomitant medications. This complication, however, is inherent to research on the corticosteroid-treated patients. Third, the assessment period of 24 weeks was relatively brief. With longer lamotrigine treatment, greater changes in amygdala volume and perhaps HAM-D and YMRS scores might have been observed. Fourth, our findings from corticosteroid-treated patients may or may not be generalizable to amygdala atrophy in other excess corticosteroid conditions, such as classic congenital adrenal hyperplasia12, Cushing’s syndrome13, unmedicated depression16 or bipolar disorder.17 Fifth, the relatively low baseline levels of mania and depression in our sample limited our ability to detect changes in these mood symptoms. Six, given the exploratory nature of the data analysis corrections for multiple comparison were not performed potentially increasing the possibility of Type I error.
In summary, this study suggests that lamotrigine may attenuate amygdala volume atrophy in patients on chronic corticosteroids. Research with animal models is needed to explain the mechanism by which lamotrigine buffers the atrophic effect of corticosteroids on the amygdala, although reduction in glutamate is a possible explanation. Further investigation with a larger sample size of corticosteroid-patients and longer assessment period is required to establish whether continuous lamotrigine treatment persistently improves amygdala volume and mood.
Acknowledgments
FUNDING: The study was supported by NIH grant MH01725 and an investigator-initiated grant from Glaxo-Smith Kline.
Footnotes
FINANCIAL DISCLOSURES: ES Brown has received research grants from NIAAA, NIDA, NIMH, AstraZeneca, Stanley Medical Research Institute, Glaxo-Smith Kline, Forest Laboratories, and McNeil. MU Shad has an investigator-initiated trial from Eli Lilly, is on the speaker panel for Pfizer and Bristol-Myers Squibb, and has a K23 award from NIMH. He has also served as a co-investigator for studies sponsored by Abbott Laboratories, Bristol-Myers Squibb, Hoechst-Roussell, Organon, Pfizer Inc., Wyeth-Ayerst, Upjohn, Janssen, Merck and Lundbeck. S Khanani and S Desai have reported no financial interest or conflicts of interest.
REFERENCES
- 1.Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375–382. doi: 10.1046/j.1524-4741.1999.98085.x. [DOI] [PubMed] [Google Scholar]
- 2.Bremner JD, McCaffery P. The neurobiology of retinoic acid in affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):315–331. doi: 10.1016/j.pnpbp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huffman JC, Stern TA. Neuropsychiatric consequences of cardiovascular medications. Dialogues Clin Neurosci. 2007;9(1):29–45. doi: 10.31887/DCNS.2007.9.1/jchuffman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus: Treatment and preventive therapy. Annals of the New York Academy of Sciences. doi: 10.1111/j.1749-6632.2009.04981.x. [In press] [DOI] [PubMed] [Google Scholar]
- 6.Brown ES, Suppes T, Khan DA, et al. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55–61. doi: 10.1097/00004714-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Brown ES, D JW, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55(5):538–545. doi: 10.1016/j.biopsych.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Newcomer JW, Selke G, Melson AK, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56(6):527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- 9.Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol Psychiatry. 1992;32(9):756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 10.Vyas A, Mitra R, Shankaranarayana Rao BS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105(14):5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merke DP, Fields JD, Keil MF, et al. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88(4):1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- 13.Merke DP, Giedd JN, Keil MF, et al. Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. J Clin Endocrinol Metab. 2005;90(5):2531–2536. doi: 10.1210/jc.2004-2488. [DOI] [PubMed] [Google Scholar]
- 14.Brown ES, Woolston DJ, Frol AB. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705–709. doi: 10.1016/j.biopsych.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karl A, Schaefer M, Malta LS, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13(11):993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosso IM, Killgore WD, Cintron CM, et al. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biol Psychiatry. 2007;61(6):743–749. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 19.Magarinos AM, McEwen BS, Flugge G, et al. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16(10):3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown ES, Stuard G, Liggin JD, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biol Psychiatry. 2005;57(5):543–548. doi: 10.1016/j.biopsych.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Falk WE, Mahnke MW, Poskanzer DC. Lithium prophylaxis of corticotropin-induced psychosis. JAMA. 1979;241(10):1011–1012. [PubMed] [Google Scholar]
- 22.Brown ES, Vazquez M, Nakamura A. Randomized, placebo-controlled, crossover trial of memantine for cognitive changes with corticosteroid therapy. Biol Psychiatry. 2008;64(8):727–729. doi: 10.1016/j.biopsych.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Brown ES, Wolfshohl J, Shad MU, et al. Attenuation of the effects of corticosteroids on declarative memory with lamotrigine. Neuropsychopharmacology. 2008;33(10):2376–2383. doi: 10.1038/sj.npp.1301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrese JR, Shelton MD, Rapport DJ, et al. Long-term treatment of bipolar disorder with lamotrigine. J Clin Psychiatry. 2002;63 Suppl 10:18–22. [PubMed] [Google Scholar]
- 25.Magnotta VA, Harris G, Andreasen NC, et al. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 26.Keshavan MS, Dick E, Mankowski I, et al. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002;58(2–3):173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]