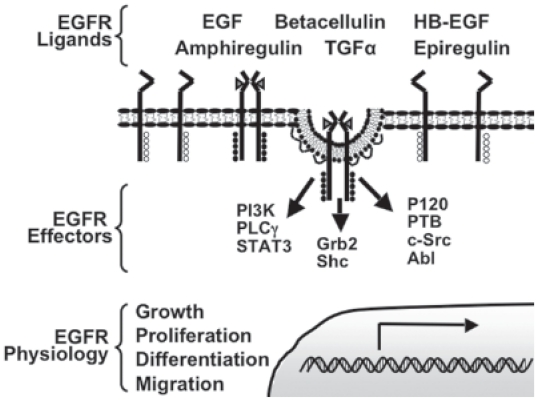
Figure 1. Schematic of EGFR activation.
Inactive EGFR exist as monomers on the plasma membrane. Upon binding of one of six endogenous ligands, two monomers dimerize and activate the receptor’s intrinsic kinase domain. The active kinase domain of one EGFR monomer transphosphorylates tyrosine residues on carboxyl terminus of its receptor pair. Once activated, the phosphotyrosines serve as docking site for downstream effectors, which include enzymes, adaptor proteins, and other regulatory molecules. Signaling from effectors integrates to modulate cell physiology, some of which are indicated. Phosphatidyl inositol 3-kinase (PI3K), phospholipase Cγ (PLCγ), signal transducers and activators of transcription 3 (STAT3), Growth factor receptor-bound protein 2 (Grb2), Src homology containing protein (Shc), p120 ras GTPase activating protein (P120), phosphatase B (PTB), cellular sarcoma (c-Src), and Abl.