Abstract
Based on phylogenetic analysis of sequence data, Aspergillus section Usti includes 21 species, inclucing two teleomorphic species Aspergillus heterothallicus (= Emericella heterothallica) and Fennellia monodii. Aspergillus germanicus sp. nov. was isolated from indoor air in Germany. This species has identical ITS sequences with A. insuetus CBS 119.27, but is clearly distinct from that species based on β-tubulin and calmodulin sequence data. This species is unable to grow at 37 °C, similarly to A. keveii and A. insuetus. Aspergillus carlsbadensis sp. nov. was isolated from the Carlsbad Caverns National Park in New Mexico. This taxon is related to, but distinct from a clade including A. calidoustus, A. pseudodeflectus, A. insuetus and A. keveii on all trees. This species is also unable to grow at 37 °C, and acid production was not observed on CREA. Aspergillus californicus sp. nov. is proposed for an isolate from chamise chaparral (Adenostoma fasciculatum) in California. It is related to a clade including A. subsessilis and A. kassunensis on all trees. This species grew well at 37 °C, and acid production was not observed on CREA. The strain CBS 504.65 from soil in Turkey showed to be clearly distinct from the A. deflectus ex-type strain, indicating that this isolate represents a distinct species in this section. We propose the name A. turkensis sp. nov. for this taxon. This species grew, although rather restrictedly at 37 °C, and acid production was not observed on CREA. Isolates from stored maize, South Africa, as a culture contaminant of Bipolaris sorokiniana from indoor air in Finland proved to be related to, but different from A. ustus and A. puniceus. The taxon is proposed as the new species A. pseudoustus. Although supported only by low bootstrap values, F. monodii was found to belong to section Usti based on phylogenetic analysis of either loci BLAST searches to the GenBank database also resulted in closest hits from section Usti. This species obviously does not belong to the Fennellia genus, instead it is a member of the Emericella genus. However, in accordance with the guidelines of the Amsterdam Declaration on fungal nomenclature (Hawksworth et al. 2011), and based on phylogenetic and physiological evidence, we propose the new combination Aspergillus monodii comb. nov. for this taxon. Species assigned to section Usti can be assigned to three chemical groups based on the extrolites. Aspergillus ustus, A. granulosus and A. puniceus produced ustic acid, while A. ustus and A. puniceus also produced austocystins and versicolorins. In the second chemical group, A. pseudodeflectus produced drimans in common with the other species in this group, and also several unique unknown compounds. Aspergillus calidoustus isolates produced drimans and ophiobolins in common with A. insuetus and A. keveii, but also produced austins. Aspergillus insuetus isolates also produced pergillin while A. keveii isolates produced nidulol. In the third chemical group, E. heterothallica has been reported to produce emethallicins, 5'-hydroxyaveranthin, emeheterone, emesterones, 5'-hydroxyaveranthin.
Keywords: Ascomycetes, Aspergillus section Usti, ITS, calmodulin, extrolites, β-tubulin, polyphasic taxonomy
INTRODUCTION
Aspergillus ustus is a common filamentous fungus found in foods, soil and indoor air environments (Samson et al. 2004). This species was considered as a relatively rare human pathogen that can cause invasive infection in immunocompromised hosts (Weiss & Thiemke 1983, Stiller et al. 1994, Verweij et al. 1999, Nakai et al. 2002, Pavie et al. 2005, Panackal et al. 2006, Yildiran et al. 2006, Krishnan-Natesan et al. 2008, Florescu et al. 2008, Vagefi et al. 2008). However, recent studies clarified that infections attributed to A. ustus are caused in most cases by another species, A. calidoustus (Houbraken et al. 2007, Varga et al. 2008, Balajee et al. 2009, Peláez et al. 2010). This species is also common in indoor air (Houbraken et al. 2007, Slack et al. 2009) and is able to colonise water distribution systems (Hageskal et al. 2011). Other species related to A. ustus can also cause human or animal infections; A. granulosus was found to cause disseminated infection in a cardiac transplant patient (Fakih et al. 1995), while A. deflectus has been reported to cause disseminated mycosis in dogs (Jang et al. 1986, Kahler et al. 1990, Robinson et al. 2000, Schultz et al. 2008, Krockenberger et al. 2011).
Raper & Fennell (1965) classified A. ustus to the Aspergillus ustus species group (Aspergillus section Usti according to Gams et al. 1985) together with four other species: A. panamensis, A. puniceus, A. conjunctus and A. deflectus. Later, Kozakiewicz (1989) revised the taxonomy of the group, and included A. ustus, A. pseudodeflectus, A. conjunctus, A. puniceus, A. panamensis and A. granulosus in the A. ustus species group, and established the A. deflectus species group including A. deflectus, A. pulvinus and A. silvaticus based on morphological studies. Klich (1993) treated A. granulosus as member of section Versicolores, and found that A. pseudodeflectus is only weakly related to this section based on morphological treatment of section Versicolores. Peterson (2000) transferred A. conjunctus, A. funiculosus, A. silvaticus, A. panamensis and A. anthodesmis to section Sparsi. More recently, Peterson (2008) examined the relationships of the Aspergillus genus using phylogenetic analysis of sequences of four loci, and assigned 15 species to this section (see below).
We examined the evolutionary relationships among species assigned to section Usti. We have used a polyphasic taxonomic approach in order to determine the delimitation and variability of known and new species. For phenotypic analyses, macro- and micromorphology of the isolates was examined, and secondary metabolite profiles were studied. For genotypic studies, partial sequences of the β-tubulin and calmodulin genes and the ITS region of the rRNA gene cluster were analysed.
MATERIALS AND METHODS
Isolates
The strains used in this study are listed in Table 1.
Table 1.
Isolates in Aspergillus section Usti and related species examined in this study.
| Species | Strain No. | Source |
|---|---|---|
| A. amylovorus | CBS 600.67T = NRRL 5813 = IMI 129961 = VKM F-906 = IBT 23158 | Wheat starch, Ukraine |
| A. calidoustus | CBS 112452 | Indoor air, Germany |
| CBS 113228 | ATCC 38849; IBT 13091 | |
| CBS 114380 | Wooden construction material, Finland | |
| CBS 121601; 677 | Bronchoalveolar lavage fluid, proven invasive aspergillosis, Nijmegen, the Netherlands†. | |
| CBS 121610; 91 | Post-cataract surgery endophthalmitis, Turkey | |
| A. californicus | CBS 123895T = IBT 16748 | Ex chamise chaparral (Adenostoma fasciculatum), in the foothills of the San Gabriel Mountains on Baldy Mountain Road near Shinn Road Intersection, North of Claremont and near San Antonio Dam, California, USA, Jeff S. La Favre, 1978. A wildfire occurred here 31/8 1975. |
| A. carlsbadensis | CBS 123893 = IBT 16753 | Soil, Galapagos Islands, Ecuador |
| CBS 123894T = IBT 14493 | Lechuguilla Cave, Carlsbad Caverns National Park, New Mexico, USA, D.E. Northup, 1992 | |
| CBS 123903 =IBT 18616 | Soil, Carthage, Tunesia | |
| A. cavernicola | CBS 117.76T = NRRL 6327 | Soil, cave wall, Romania |
| A. deflectus | CBS 109.55T = NRRL 2206 = IBT 24665 | Soil, Rio de Janeiro, Brazil |
| NRRL 4235 = IBT 25291 | Potting soil | |
| NRRL 13131 = IBT 25254 | Unknown | |
| A. egyptiacus | CBS 123892 = IBT 16345 = RMF 9515 | Soil, Iraq |
| CBS 656.73T = NRRL 5920 | Sandy soil, under Olea europaea, Ras-El-Hikma, Egypt | |
| CBS 991.72C | Bare ferruginous soil, Dahkla Oasis, Western desert, Egypt | |
| CBS 991.72A | Bare ferruginous soil, Dahkla Oasis, Western desert, Egypt | |
| CBS 991.72B | Bare ferruginous soil, Dahkla Oasis, Western desert, Egypt | |
| CBS 991.72F | Bare ferruginous soil, Dahkla Oasis, Western desert, Egypt | |
| CBS 991.72E | Bare ferruginous soil, Dahkla Oasis, Western desert, Egypt | |
| A. elongatus | CBS 387.75T = NRRL 5176 | Alkaline Usar soil, Lucknow, India |
| A. germanicus | CBS 123887T = DTO 27-D9 = IBT 29365 | Indoor air, Stuttgart, Germany |
| A. granulosus | CBS 588.65T | Soil, Fayetteville, Arkansas, USA |
| CBS 119.58 | Soil, Texas, USA | |
| A. heterothallicus | CBS 489.65T | Soil, Costa Rica |
| CBS 488.65 | Soil, Costa Rica | |
| A. insuetus | CBS 107.25T = NRRL 279 | South Africa |
| CBS 119.27 = NRRL 4876 | Soil, Iowa, USA | |
| CBS 102278 | Subcutaneous infection, Spain | |
| A. kassunensis | CBS 419.69T = NRRL 3752 = IMI 334938 = IBT 23479 | Soil, Damascus, Syria |
| A. keveii | CBS 209.92 | Soil, La Palma, Spain |
| CBS 561.65 = NRRL 197 | Soil, Panama | |
| IBT 10524 = CBS 113227 = NRRL 1254 | Soil, Panama | |
| IBT 16751 | Soil at trail from Pelican Bay to inland, Isla Santa Cruz, Galapagos Islands, Ecuador, Tjitte de Vries and D.P. Mahoney, 1968 | |
| A. lucknowensis | CBS 449.75T = NRRL 3491 | Alkaline Usar soil, Lucknow, India |
| A. monodii | CBS 434.93 | Dung of Procavia sp. (daman), Darfur, Sudan |
| CBS 435.93T | Dung of sheep, Ennedi, Chad | |
| A. pseudodeflectus | CBS 596.65 | Sugar, USA, Louisiana |
| CBS 756.74T | Desert soil, Egypt, Western Desert | |
| NRRL 4846 = IBT 25256 | Unknown | |
| A. pseudoustus | ATCC 36063 = NRRL 5856 = CSIR 1128 = CBS 123904T = IBT 28161 | Stored maize, South Africa |
| MRC 096 = IBT 31044 | Contaminant in a Bipolaris sorokiniana strain (MRC 093), South Africa | |
| A. pseudoustus | IBT 22361 | Indoor air, Finland |
| A. puniceus | CBS 495.65T | Soil, Zarcero, Costa Rica |
| CBS 128.62 | Soil, Louisiana, USA | |
| A. subsessilis | CBS 502.65T = NRRL 4905 = IMI 135820 = IBT 23160 | Desert soil, Mojave desert, CA, USA |
| CBS 988.72 = NRRL 4907 = IMI 335782 = IBT 23165 | Desert soil, USA | |
| A. turkensis | CBS 504.65T = NRRL 4993 = WB 4993 = IBT 22553 | Soil, Turkey |
| A. ustus | CBS 116057 | Antique tapestries, Krakow, Poland |
| CBS 114901 | Carpet, The Netherlands | |
| CBS 261.67T | Culture contaminant, USA | |
| CBS 133.55 | Textile buried in soil, Netherlands | |
| CBS 239.90 | Man, biopsy of brain tumor, Netherlands | |
| CBS 113233 = IBT 14495 | Cave wall, Lechuguilla Cave, Carlsbad, New Mexico | |
| CBS 113232 = IBT 14932 | Indoor air, Denmark |
Morphological analysis
For macromorphological observations, Czapek Yeast Autolysate (CYA), Malt Extract Autolysate (MEA) agar, Yeast Extract Sucrose Agar (YES), Creatine Agar (CREA), and Oatmeal Agar (OA) were used (Samson et al. 2004). The isolates were inoculated at three points on each plate of each medium and incubated at 25 °C and 37 °C in the dark for 7 d. For micromorphological observations, microscopic mounts were made in lactic acid with cotton blue from MEA colonies and a drop of alcohol was added to remove air bubbles and excess conidia.
Extrolite analysis
The isolates were grown on CYA and YES at 25 °C for 7 d. Extrolites were extracted after incubation. Five plugs of each agar medium were taken and pooled together into same vial for extraction with 0.75 mL of a mixture of ethyl acetate/dichloromethane/methanol (3:2:1) (v/v/v) with 1 % (v/v) formic acid. The extracts were filtered and analysed by HPLC using alkylphenone retention indices and diode array UV-VIS detection as described by Frisvad & Thrane (1987), with minor modifications as described by Smedsgaard (1997).
Genotypic analysis
The cultures used for the molecular studies were grown on malt peptone (MP) broth using 1 % (w/v) of malt extract (Oxoid) and 0.1 % (w/v) bacto peptone (Difco), 2 mL of medium in 15 mL tubes. The cultures were incubated at 25 °C for 7 d. DNA was extracted from the cells using the Masterpure™ yeast DNA purification kit (Epicentre Biotechnol.) according to the instructions of the manufacturer. The ITS region and parts of the β-tubulin and calmodulin genes were amplified and sequenced as described previously (Houbraken et al. 2007, Varga et al. 2007, 2008).
Data analysis
DNA sequences were edited with the DNASTAR computer package. Alignments of the sequences were performed using MEGA v. 4 (Tamura et al. 2007). Phylogenetic analysis of sequence data was performed using PAUP v. 4.0b10 (Swofford 2000). Alignment gaps were treated as fifth character state, parsimony uninformative characters were excluded and all characters were unordered and equal weight. Maximum parsimony analysis was performed for all data sets using the heuristic search option. To assess the robustness of the topology, 1 000 bootstrap replicates were run by maximum parsimony (Hillis & Bull 1993). Other measures including tree length, consistency index and retention index (CI and RI, respectively) were also calculated. Aspergillus versicolor CBS 583.65T was used as outgroup in these analyses. Sequences were deposited at GenBank under accession numbers FJ531124–FJ531191.
RESULTS AND DISCUSSION
Phylogenetic analysis
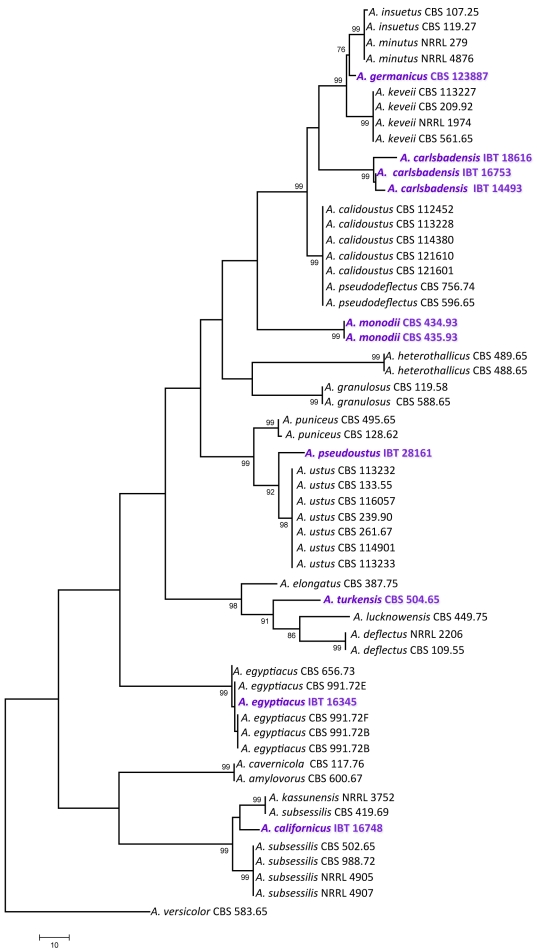
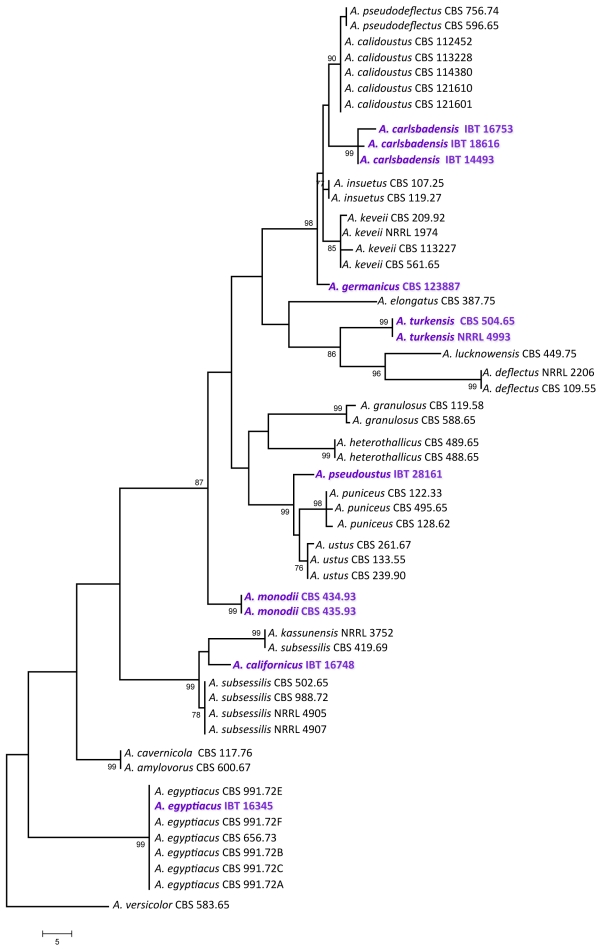
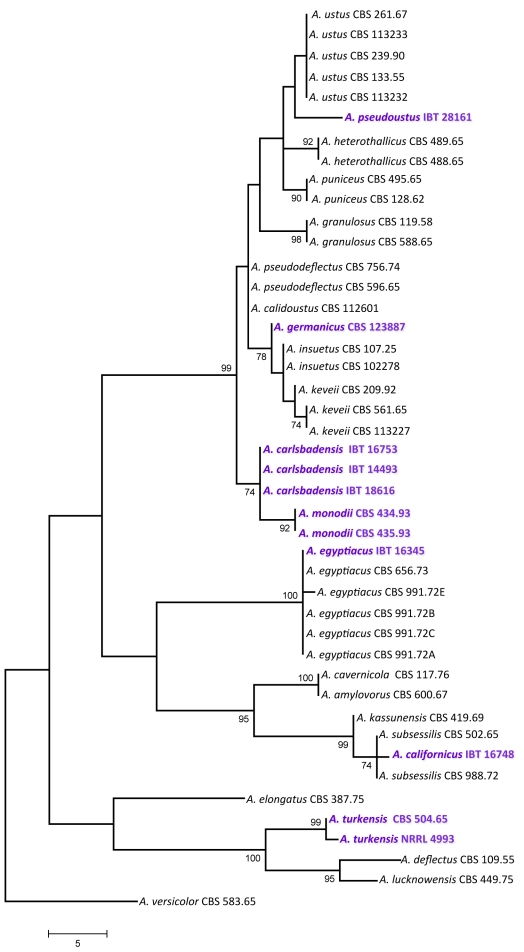
For the molecular analysis of the isolates, three genomic regions, the ITS region, and parts of the calmodulin and β-tubulin genes were amplified and sequenced. Phylogenetic analysis of the data was carried out using parsimony analysis. For the analysis of part of the β-tubulin gene, 589 characters were analysed, 197 of which were found to be parsimony informative. One of the 78 MP trees based on partial β-tubulin genes sequences is shown in Fig. 1 (tree length: 661 steps, consistency index: 0.6445, retention index: 0.8922). The calmodulin data set included 475 characters, with 266 parsimony informative characters. One of the 119 MP trees based on partial calmodulin gene sequences is shown in Fig. 2 (tree length: 890, consistency index: 0.5753, retention index: 0.8788). The ITS data set included 541 characters with 100 parsimony informative characters. One of the 8 MP trees is shown in Fig. 3 (tree length: 224, consistency index: 0.7366, retention index: 0.9230).
Fig. 1.
The single MP tree obtained based on phylogenetic analysis of β-tubulin sequence data of Aspergillus section Usti. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Fig. 2.
One of the MP trees obtained based on phylogenetic analysis of calmodulin sequence data of Aspergillus section Usti. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Fig. 3.
One of the MP trees obtained based on phylogenetic analysis of ITS sequence data of Aspergillus section Usti. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Based on phylogenetic analysis of sequence data, Aspergillus section Usti includes now 21 species, at least two of which are able to reproduce sexually: Aspergillus heterothallicus (=Emericella heterothallica) and Fennellia monodii. Although supported only by low bootstrap values, F. monodii was found to belong to section Usti based on phylogenetic analysis of either loci (Figs 1, 2, 3). BLAST searches to the GenBank database also resulted in closest hits from section Usti (A. pseudodeflectus and A. calidoustus for the ITS and calmodulin sequence data, and A. ustus and A. insuetus for the β-tubulin sequences). Fennellia monodii was described in 1990 by Locquin-Linard from dung of herbivores in Tchad and Sudan. This species is characterised by two-valved ascospores with low, wrinkled equatorial crests. The anamorph of this species has not yet been observed in spite of repeated attempts using various media (data not shown). This species obviously does not belong to the Fennellia genus, instead it is a member of the Emericella genus. However, in accordance with the guidelines of the Amsterdam Declaration on fungal nomenclature (Hawsksworth et al. 2011), and based on phylogenetic and physiological evidence, we propose the new combination Aspergillus monodii comb. nov. for this interesting species.
Another new species in this section was isolated from indoor air in Germany. This species has identical ITS sequences with A. insuetus CBS 119.27, but is clearly distinct from that species based on β-tubulin and calmodulin sequence data. This species is unable to grow at 37 °C, similarly to A. keveii and A. insuetus. We propose the name A. germanicus sp. nov. for this taxon.
Isolate IBT 16753 from Galapagos Islands, Ecuador, and IBT 14493 isolated from Lechuguilla Cave, Carlsbad Caverns National Park in New Mexico, USA were found to be related to, but clearly distinct from a clade including A. calidoustus, A. pseudodeflectus, A. insuetus and A. keveii on all trees. This species is also unable to grow at 37 °C, and acid production was not observed on CREA. We propose the name A. carlsbadensis sp. nov. for this taxon.
Isolate IBT 16748 was isolated from chamise chaparral (Adenostoma fasciculatum) in California, USA in 1978. It was found to be related to a clade including A. subsessilis and A. kassunensis on all trees. This species grew well at 37 °C, and acid production was not observed on CREA. We propose the name A. californicus sp. nov. for this taxon.
The “A. deflectus” isolate CBS 504.65 came from soil in Turkey is clearly distinct from the A. deflectus type strain on all trees, indicating that this isolate represents a distinct species in this section. This species grew, although rather restrictedly at 37 °C, and acid production was not observed on CREA. We propose the name A. turkensis sp. nov. for this taxon.
Another new species in this section, tentatively called A. pseudoustus sp. nov., is represented by NRRL 5856 = IBT 28161, which was found to be related to, but clearly different from A. ustus and A. puniceus on all trees (Figs 1, 2, 3). This isolate came from stored maize, South Africa. Other isolates belonging to this species include a culture contaminant of Bipolaris sorokiniana from South Africa (IBT 31044), and one isolate came from indoor air in Finland (IBT 22361).
Isolate IBT 16345 from soil, Iraq is a new isolate of A. egyptiacus based on all sequence data. The isolate grew well at 37 °C, and acid production was not observed on CREA. This is the first isolate of this species which was isolated outside Egypt.
In agreement with the data of Peterson (2008), A. kassunensis, which was treated as a synonym of A. subsessilis (Samson 1979, Samson & Moucchaca 2004), is also a valid species, related to A. subsessilis and A. californicus (Figs 1, 2, 3). Aspergillus cavernicola was treated as a synonym of A. varians by Samson (1979); however, based on sequence data, it is conspecific with A. amylovorus and belongs to section Usti, while the A. varians type strain belongs to Aspergillus section Nidulantes (data not shown). Aspergillus amylovorus was invalidly described (nom. inval., Art. 37) from wheat starch (Panasenko 1964), and subsequently validated by Samson (1979), while A. cavernicola was described in 1969 from cave wall from Romania. This species was validly described and hence is the correct name for A. cavernicola (= A. amylovorus).
Extrolites
The mycotoxins and other secondary metabolites found to be produced by the examined species in this study are listed in Table 2. Species assigned to section Usti could clearly be assigned to three chemical groups based on the extrolites produced by them. Aspergillus ustus, A. granulosus and A. puniceus produced ustic acids in common. Aspergillus ustus and A. puniceus also produced austocystins and versicolorins. In the second chemical group, A. pseudodeflectus produced drimans (Hayes et al. 1996) in common with the other species in this group, and also several unique unknown compounds. Aspergillus calidoustus isolates produced drimans and ophiobolins (Cutler et al. 1984) in common with A. insuetus and A. keveii, but also produced austins (Chexal et al. 1976) not identified in other species of section Usti. Aspergillus insuetus isolates also produced pergillin (Cutler et al. 1980), while A. keveii isolates produced nidulol. In the third chemical group, E. heterothallica has been reported to produce emethallicins A–F (Kawahara et al. 1989, 1990a, b), 5'-hydroxyaveranthin (Yabe et al. 1991), emeheterone (Kawahara et al. 1988), emesterones A & B (Hosoe et al. 1998), 5'-hydroxyaveranthin (Yabe et al. 1991), Mer-NF8054X (Mizuno et al. 1995). This latter compound, an 18,22-cyclosterol derivative, is closely related to the emesterones, and was also identified in an isolate identified as A. ustus (Mizuno et al. 1995). Aspergillus deflectus produces several antibiotics, including desferritriacetylfusigen, which inhibits the growth of bacteria (Anke 1977), and deflectins, angular azaphilons, which have antibiotic properties, and exhibit lytic activities against bacteria and erythrocytes (Anke et al. 1981). Aspergillus egyptiacus has been suggested to be more closely related to E. nidulans than to A. versicolor based on its biochemical behavior (Zohri & Ismail 1994). Aspergillus egyptiacus produces fumitremorgins and verruculogen, thus resembling A. caespitosus in that aspect. However A. caespitosus is placed within Aspergillus section Nidulantes (Peterson 2008, J. Varga, unpubl. data). Aspergillus elongatus CBS 387.75 produced fumitremorgin C, but other fumitremorgins and verruculogen could not be detected in that strain. The same strain also produced a member of the norgeamide / notoamide / aspergamide / stephacidin family of secondary metabolites (notoamide E). This type of compound has also been found in a strain of A. versicolor (Greshock et al. 2008).
Table 2.
Extrolites produced by species assigned to Aspergillus section Usti.
| Species | Extrolites produced |
|---|---|
| A. amylovorus | An asperugin, monascorubramin-like extrolites, (CANO, SCYT, SENSTER, STARM) |
| A. calidoustus | Austins, drimans, ophiobolins G and H, TMC-120B, (ALTIN, FAAL, KNOF) |
| A. californicus | An arugosin, (CANDU, SAERLO, SCAM, SEND, XANXU) |
| A. carlsbadensis | Brevianamide A (only in IBT 14493), [An arugosin, DRI, TRITRA, TIDL (not in IBT 16753), GNI (only in IBT 18616), EMO (only in IBT 14493)] |
| A. deflectus | Desferritriacetylfusigen, deflectins A & B, emerin, a shamixanthone, (FUMU, RED2) |
| A. egyptiacus | Fumitremorgin A, fumitremorgin B, verruculogen, (FYEN, UTSCABI, TOPLA, FUMU, PRUD, HØJV) |
| A. elongatus | Fumitremorgin C, notoamide E, (DYK, SENT, TERRET) |
| A. germanicus | Drimans, (DRUL, KNAT, SLOT, SNOF) |
| A. granulosus | Asperugins, ustic acids, nidulol, drimans, (KMET, PUBO, SENSTER, SFOM) |
| A. heterothallicus | Emethallicins A, B, C, D, E & F, emeheterone, emesterones A & B and Mer-NF8054X, 5′-hydroxyaveranthin, stellatin, sterigmatocystin, (DRI, NIDU) |
| A. insuetus | Asperugins, drimans, ophiobolins G and H, pergillin-like compound, (AU, HETSCYT, INSU) |
| A. kassunensis | Asperugins, Mer-NF8054X, (FYRT, SAERLO, SENSCAB, SENSTER) |
| A. keveii | Asperugins, drimans, ophiobolins G and H, nidulol, (DRI, HETSCYT, INSU, PUBO, SENSTER, UP) |
| A. lucknowensis | An arugosin, (GULT, PULK, RED1) |
| A. monodii | Terrein, (DYVB, METK) |
| A. pseudodeflectus | Drimans, (DRI, DRUL, HUT, SLOT), asperugins in NRRL 4846 |
| A. pseudoustus | Asperugins, austamide, prolyl-2-(1′,1′-dimethylallyl) tryptophyldiketopiperazine, 12,13-dihydroaustamide, 12,13-dehydroprolyl-2-(1′,1′-dimetylallyl)-tryptophyldiketopiperazine, 10,20-dehydro[12,13-dehydropropyl-2-1′,1′-dimethylallyl)tryptophyldiketopiperazine], 12,13-dihydro-12-hydroxyaustamide, austdiol, dihydrodeoxy-8-epi-austdiol, austocystin A, B, C, D, E, F, G, H, I, norsolorinic acid, versicolorin C, averufin, (DRI, HETSCYT, SENSTER, UZ) |
| A. puniceus | Ustic acids, austocystins (and versicolorins), phenylahistin, nidulol, (SENSTER) |
| A. subsessilis | Mer-NF8054X, (SENSCAB, VIRO) |
| A. turkensis | An austocystin, deflectins, emerin, a shamixanthone, (RED2) |
| A. ustus | Ustic acids, austocystins (and versicolorins), austalides, nidulol, (SENSTER) |
All designations in parenthesis with capital letters are secondary metabolites with characteristic chromophores (UV spectra) and retention-times, but their chemical structure is not yet known.
Of particular interest is A. pseudoustus NRRL 5856 = CSIR 1128, which was originally identified as A. ustus and the first strain from which austamides, austdiols and austocystins (Table 2) were isolated (Steyn 1971, 1973, Steyn & Vleggaar 1974, 1976a, b, Vleggaar et al. 1974). This very toxic species has, however, only been isolated from maize in South Africa twice, and once in indoor air in Finland. All three strains examined produced austamides, austdiol and austocystins. The austocystins have been found in A. ustus, A. puniceus and A. pseudoustus and one austocystin has also been found in A. turkensis. The austocystins seem to be another biosynthetic family of secondary metabolites that are derived from the versicolorins. In other species in sections Aenei, Versicolores and Nidulantes, versicolorins are precursors of sterigmatocystin and in few species, the aflatoxins (Frisvad et al. 2005, Varga et al. 2009). Sterigmatocystin has not yet been found in any species in section Usti, but a related metabolite, listed as SENSTER in Table 2 is common in this section, and may be related to sterigmatocystin, as it has a similar UV spectrum.
Comparing the secondary metabolite profiles of section Usti with other sections within subgenus Nidulantes, nidulol, and versicolorins are also produced by members of sections Versicolores and Nidulantes (Cole & Schweikert 2003). Interestingly, versicolorin, sterigmatocystin and 5'-hydroxyaveranthin are intermediates of the aflatoxin biosynthetic pathway and also produced by species assigned to Aspergillus sections Flavi and Ochraceorosei (Yabe et al. 1991, Frisvad et al. 2005). Other extrolites found in species in section Usti are also found in other sections in subgenus Nidulantes: arugosins, asperugins, austins and the metabolite DRI are present in species of the different sections. On the other hand, several metabolites have only been found in section Usti, including austamide, austdiol, austocystins, deflectins, drimans, emethallicins, emeheterones and ustic acids (Table 2). Two species produce red pigments, A. amylovorus produce a large number of monascorubramin like red pigments, while A. turkensis produce few monascorubramin-like extrolites.
Species descriptions
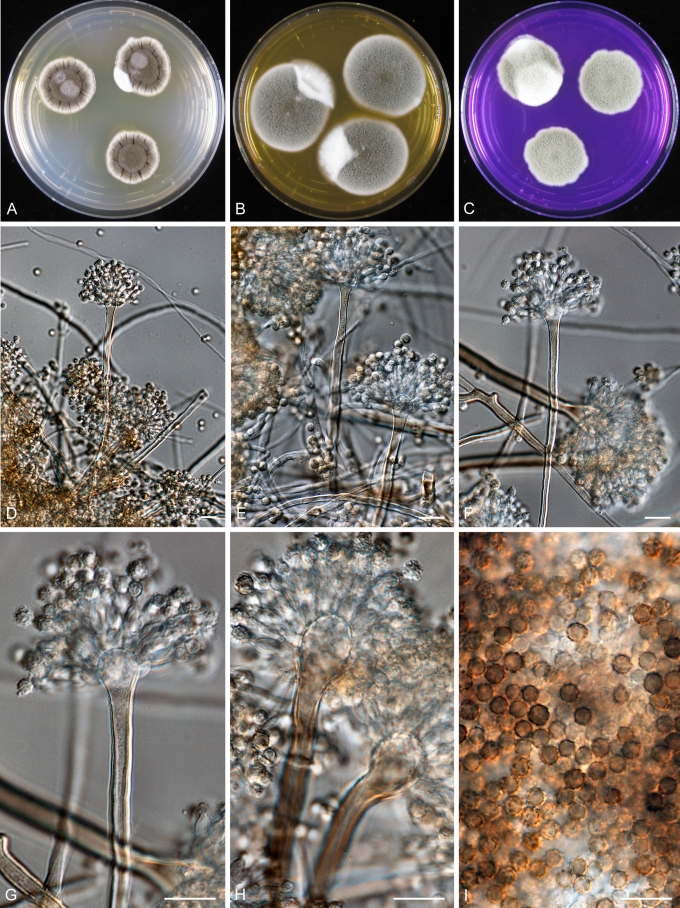
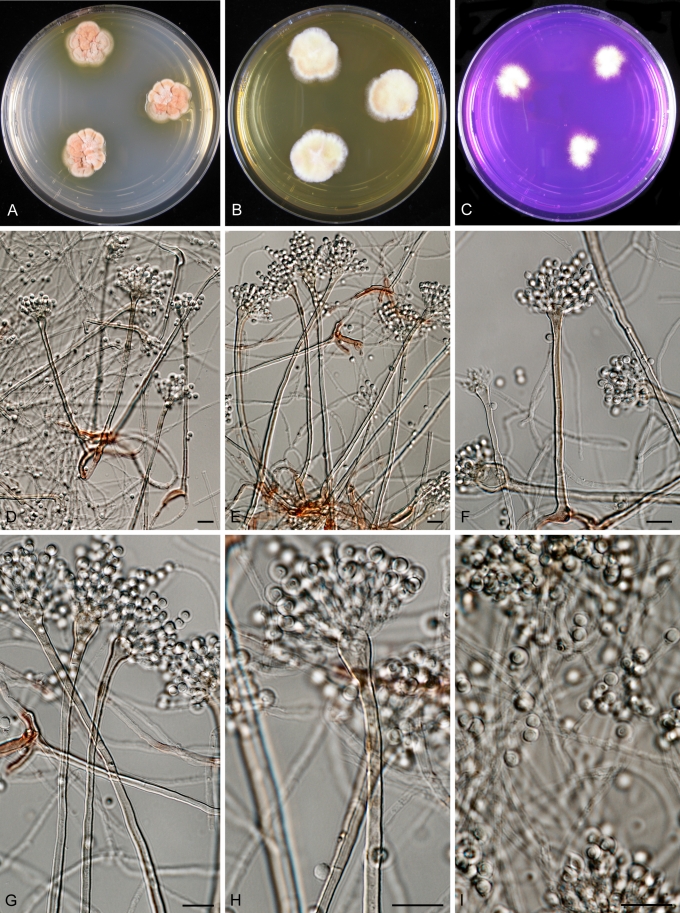
Aspergillus carlsbadensis Frisvad, Varga & Samson, sp. nov. MycoBank MB560399 Fig. 4.
Fig. 4.
Aspergillus carlsbadensis Frisvad, Varga & Samson sp. nov. A–C. Colonies incubated at 25 °C for 7 d, A. CYA, B. MEA, C. Tufts of Hülle cells. D–E, G–I. Conidiophores and conidia. F. Hülle cells. Scale bars = 10 μm.
Coloniis flavo-brunneis, cum caespitulis ex conglomerationibus cellularum obtegentium (“Hülle”). Cellulis obtegentibus (“Hülle”) hyalinis, crassitunicatis, globosis vel late ellipsoideis, 15–30 μm. Conidiophoris biseriatis, stipitibus plerumque levibus, brunneis, 4–5 μm latis. Vesiculis globosis, 10–14 μm diam. Conidiis conspicue ornamentatis, echinulatis vel verrucosis, ellipsoideis, 2.5–3.0 × 3.0–3.5 μm.
Typus: USA, from soil, Lechuguilla Cave, Carlsbad Caverns National Park, New Mexico, isolated by D.E. Northup, 1992, (CBS H-30634 -- holotypus, culture ex-type CBS 123894).
CYA, 1 wk, 25 °C: 30–32 mm (poor to medium sporulation, cream yellow to dark brown reverse, Hülle cells), MEA, 1 wk, 25 °C: 7–29 mm (rather poor sporulation, light yellow to cream reverse), YES, 1 wk, 25 °C: 35–45 mm (no sporulation, yellow to curry yellow), OA, 1 wk, 25 °C: 25–32 mm (Hülle cells), CYA, 1 wk, 37 °C: no growth, CREA: good growth (18–22 mm) and no acid production.
Colonies yellow brown with white tufts of conglomerates of Hülle cells. Hülle cells hyaline, thick-walled, globose to broadly ellipsoidal, 15–30 μm. Conidiophores biseriate with typical smooth-walled, brown, 4–5 μm wide stipes. Vesicles globose, 10–14 μm in diam. Conidia, distinctly ornamented with spines or warts, ellipsoidal 2.5–3.0 × 3.0–3.5 μm.
The taxon is related to, but clearly distinct from a clade including A. calidoustus, A. pseudodeflectus, A. insuetus and A. keveii on all trees. This species is also unable to grow at 37 °C, and acid production was not observed on CREA.
Aspergillus californicus Frisvad, Varga & Samson, sp. nov. MycoBank MB560400. Fig. 5.
Fig. 5.
Aspergillus californicus Frisvad, Varga & Samson sp. nov. A–C. Colonies incubated at 25 °C for 7 d, A. CYA, B. MEA, C. CREA, D–I. Conidiophores and conidia. Scale bars = 10 μm.
Coloniis clare flavis, cum caespitulis albidis ex conglomerationibus cellularum obtegentium (“Hülle”). Cellulis obtegentibus (“Hülle”) hyalinis, crassitunicatis, globosis vel late ellipsoideis. Conidiophoris biseriatis, stipitibus levibus, clare brunneis, 3.5–5 μm latis. Vesiculis globosis,. 11–16 μm in diam. Conidiis levibus vel subtiliter exasperates, subglobosis vel globosis, hyalinis vel viridibus, 2.5–3.0 μm.
Typus: USA, foothills of San Gabriel Mountains, California, ex chamise chaparral (Adeonostoma fasciculatum), Jeff S. La Favre, 1978 (CBS H-20635 -- holotypus, culture ex-type CBS 123895).
CYA, 1 wk, 25 °C: 18–20 mm (poor sporulation, yellow brown reverse, Hülle cells), MEA, 1 wk, 25 °C: 6–9 mm (rather poor sporulation, yellow brown reverse), YES, 1 wk, 25 °C: 23–26 mm (no sporulation, cream yellow reverse), OA, 1 wk, 25 °C: 18–21 mm (Hülle cells), CYA, 1 wk, 37 °C: no growth, CREA: good growth and no acid production.
Colonies light yellow with white tufts of conglomerates of Hülle cells. Hülle cells hyaline, thick-walled, globose to broadly ellipsoidal, 25–50 μm. Conidiophores biseriate with smooth-walled, light brown, 3.5–5 μm wide stipes. Vesicles globose, 11–16 μm in diam. Conidia, smooth to finely roughened, subglobose to globose, hyaline to greenish, 2.5–3.0 μm.
This species grew well at 37 °C, and acid production was not observed on CREA. It was found to be related to species in a clade including A. subsessilis and A. kassunensis.
Aspergillus germanicus Varga, Frisvad & Samson, sp. nov. MycoBank MB560401. Fig. 6.
Fig. 6.
Aspergillus germanicus Varga, Frisvad & Samson sp. nov. A–C. Colonies incubated at 25 °C for 7 d, A. CYA, B. MEA, C. Tufts of Hülle cells. D–E, G–I. Conidiophores and conidia. F. Hülle cells. Scale bars = 10 μm.
Coloniis in agaro CYA brunneis et in agaro MEA griseo-brunneis, cellulis tectegentibus (“Hülle”) nullis. Conidiophoris biseriatis, stipitibus plerumque levibus, brunneis, 6–9 μm latis. Vesiculis spathuliformibus, 14–22 μm diam. Conidiis conspicue echinulatis, globosis, brunneis, 3.5–5.0 μm diam.
Typus: Germany, ex indoor air, Stuttgart. Isolated by U. Weidner (CBS H-20636 -- holotypus, culture ex-type CBS 123887).
CYA, 1 wk, 25 °C: 22–26 mm (poor to medium sporulation, yellow brown to orange reverse, pigment diffusing, Hülle cells), MEA, 1 wk, 25 °C: 12–16 mm (good sporulation, light yellow to cream reverse), YES, 1 wk, 25 °C: 32–37 mm (some sporulation, yellow brown reverse), OA, 1 wk, 25 °C: 28–32 mm, CYA, 1 wk, 37 °C: 7–9 mm, CREA: good growth and no acid production.
Colonies on CYA brown, on MEA greyish brown. Hülle cells not observed. Conidiophores biseriate with typical smooth-walled, brown, 6–9 μm wide stipes. Vesicles spathulate, 14–22 μm diam. Conidia, distinctly echinulate, globose, brown, 3.5–5.0 μm.
This species has identical ITS sequences with A. insuetus CBS 119.27, but is clearly distinct from that species based on β-tubulin and calmodulin sequence data.
Aspergillus monodii (Locquin-Linard) Varga, Frisvad & Samson, comb. nov. MycoBank MB560402. Fig. 7.
Fig. 7.
Aspergillus monodii (Locquin-Linard) Varga, Frisvad & Samson comb. nov. A–B. Stromata containing ascomata, grown at 25 °C for 7 d, C. Mycelium with ascoma initials. D. Hülle cells, E–G. Asci and ascospores. Scale bars = 10 μm.
Basionym: Fennellia monodii Locquin-Linard, Mycotaxon 39: 10, 1990.
CYA, 1 wk, 25 °C: 2–21 mm (no sporulation, white to cream reverse), MEA, 1 wk, 25 °C: 6–8 mm (ascomata, light yellow reverse), YES, 1 wk, 25 °C: 8–23 mm (no sporulation, yellow to red brown reverse, yellow obverse), OA, 1 wk, 25 °C: 9–19 mm (ascomata), CYA, 1 wk, 37 °C: 0–2 mm, CREA: poor growth and no acid production.
Colonies producing an orange brown crusts of stromata with ascomata 200–350 μm in diam. Hülle cells forming the structure of the stromata, globose to ellipsoidal, 8–40 μm diam. Asci 8–10 × 10–13 μm. Ascospores 3.0–3.5 × 4.5–5.0 μm, hyaline, smooth-walled with two equatorial rings. Aspergillus anamorph not observed on various media and after cultivation at different temperatures.
This species occurs on dung and found on sheep dung in Chad and daman dung in Soudan.
Aspergillus pseudoustus Frisvad, Varga & Samson, sp. nov. MycoBank MB560403. Fig. 8.
Fig. 8.
Aspergillus pseudoustus Frisvad, Varga & Samson sp. nov. A–C. Colonies incubated at 25 °C for 7 d, A. CYA, B. MEA, C. CREA, D–I. Conidiophores and conidia. Scale bars = 10 μm.
Coloniis in agaro CYN cinnamomeo-brunneis et in agaro MEA flavo-brunneis, cellulis obtegentibus (“Hülle”) nullis. Conidiophoris biseriatis, stipitibus plerumque levibus, brunneis, 3.5–5 μm latis. Vesiculis globosis, 10–14 μm diam. Conidiis levibus vel distinct echinulatis, globosis, brunneis vel viridibus, 2.5–3.0 μm.
Typus: South Africa, ex stored maize (CBS H-20637 -- holotypus, culture ex-type CBS 123904).
CYA, 1 wk, 25 °C: 30–32 mm (medium sporulation, yellow brown reverse), MEA, 1 wk, 25 °C: 15–25 mm (rather poor sporulation, light yellow reverse), YES, 1 wk, 25 °C: 35–45 mm (no sporulation, curry yellow to brown reverse), OA, 1 wk, 25 °C: 30–36 mm, CYA, 1 wk, 37 °C: no growth, CREA: 28–34 mm, no acid production.
Colonies on CYA cinnamon brown, on MEA yellow brown. Hülle cells not observed. Conidiophores biseriate with typical smooth-walled, brown, 3.5–5 μm wide stipes. Vesicles globose, 10–14 μm in diam. Conidia, smooth to distinctly echinulate, globose, brown to greenish, 2.5–3.0 μm.
Other strains: MRC 096 = IBT 31044, contaminant in Bipolaris sorokiniana, isolated from maize, South Africa; IBT 22361, indoor air, Finland
Aspergillus pseudoustus sp. nov., is related to, but clearly different from A. ustus and A. puniceus on all trees. This isolate came from stored maize, South Africa. Other isolates belonging to this species include a culture contaminant of Bipolaris sorokiniana from South Africa (IBT 31044), and one isolate came from indoor air in Finland (IBT 22361).
Aspergillus turkensis Varga, Frisvad & Samson sp. nov. MycoBank MB560404. Fig. 9.
Fig. 9.
Aspergillus turkensis Varga, Frisvad & Samson sp. nov. A–C. Colonies incubated at 25 °C for 7 d, A. CYA, B. MEA, C. CREA, D–I. Conidiophores and conidia. Scale bars = 10 μm.
Coloniis in agaro CYN clare brunneis et in agaro MEA flavo-brunneis, cellulis obtegentibus (“Hülle”) nullis. Conidiophoris minute biseriatis, stipitibus plerumque levibus, clare brunneis, 2.5–3 μm latis. Vesiculis spathuliformibus, 5–8 μm diam. Conidiis levibus, globosis, hyalinis, 2.5–3.0 μm diam.
Typus: Turkey, ex soil isolated by K.B. Raper in 1950 (CBS H-20638 -- holotypus, culture ex-type CBS 504.65).
CYA, 1 wk, 25 °C: 13–18 mm (poor sporulation, red orange reverse), MEA, 1 wk, 25 °C: 4–10 mm (rather poor sporulation, cream yellow reverse), YES, 1 wk, 25 °C: 35–45 mm (no sporulation, orange yellow reverse, yellow obverse), OA, 1 wk, 25 °C: 14–17 mm (yellow reverse and obverse), CYA, 1 wk, 37 °C: 6–14 mm, CREA: weak growth and no acid production.
Colonies on CYA light brown, on MEA pale yellow brown. Hülle cells not observed. Conidiophores small biseriate with typical smooth-walled, light brown, 2.5–3 μm wide stipes. Vesicles spathulate, 5–8 μm diam. Conidia, smooth-walled, globose, hyaline, 2.5–3.0 μm.
Isolate CBS 504.65 is distinct from the A. deflectus ex-type strain on all trees, indicating that this isolate represents a distinct species in this section. This species grew, although rather restrictedly at 37 °C, and acid production was not observed on CREA.
Acknowledgments
We thank Uwe Braun for the Latin diagnosis and advice on nomenclatural issues.
Footnotes
Taxonomic novelties: Aspergillus carlsbadensis Frisvad, Varga & Samson sp. nov., Aspergillus californicus Frisvad, Varga & Samson sp. nov., Aspergillus germanicus Varga, Frisvad & Samson sp. nov., Aspergillus monodii (Locquin-Linard) Varga, Frisvad & Samson comb. nov., Aspergillus pseudoustus Frisvad, Varga & Samson sp. nov., Aspergillus turkensis Varga, Frisvad & Samson sp. nov.
REFERENCES
- Alastruey-Izquierdo A, Cuesta I, Houbraken J, Cuenca-Estrella M, Monzón A, Rodriguez-Tudela JL. (2010). In vitro activity of nine antifungal agents against clinical isolates of Aspergillus calidoustus. Medical Mycology 48: 97–102 [DOI] [PubMed] [Google Scholar]
- Anke H. (1977). Metabolic products of microorganisms. 163. Desferritriacetylfusigen, an antibiotic from Aspergillus deflectus. Journal of Antibiotics 30: 125–128 [DOI] [PubMed] [Google Scholar]
- Anke H, Kemmer T, Höfle G. (1981). Deflectins, new antimicrobial azaphilones from Aspergillus deflectus. Journal of Antibiotics 34: 923–928 [DOI] [PubMed] [Google Scholar]
- Baddley JW, Marr KA, Andes DR, Walsh TJ, Kauffman CA, et al. (2009). Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. Journal of Clinical Microbiology 47: 3271–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balajee SA, Kano R, Baddley JW, Moser SA, Marr KA, et al. (2009). Molecular identification of Aspergillus species collected for the Transplant-Associated Infection Surveillance Network. Journal of Clinical Microbiology 47: 3138–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chexal KK, Spinger JP, Clardy J, Cole RJ, Kirksey JW, et al. (1976). Austin, a novel polyisoprenoid mycotoxin from Aspergillus ustus. Journal of the American Chemical Society 98: 6748 [DOI] [PubMed] [Google Scholar]
- Cole RJ, Schweikert MA. (2003). Handbook of secondary fungal metabolites. Vols. 1–3 Amsterdam: Elsevier Academic Press; [Google Scholar]
- Cutler HG, Crumley FG, Springer JP, Cox RH, Cole RJ, et al. (1980). Pergillin: a nontoxic fungal metabolite with moderate plant growth inhibiting properties from Aspergillus ustus. Journal of Agricultural and Food Chemistry 28: 989–991 [Google Scholar]
- Cutler HG, Crumley FG, Cox RH, Springer JP, Arrendale RF, et al. (1984). Ophiobolins G and H: new fungal metabolites from a novel source, Aspergillus ustus. Journal of Agricultural and Food Chemistry 32: 778–782 [Google Scholar]
- Fakih MG, Barden GE, Oakes CA, Berenson CS. (1995). First reported case of Aspergillus granulosus infection in a cardiac transplant patient. Journal of Clinical Microbiology 33: 471–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florescu DF, Iwen PC, Hill LA, Dumitru I, Quader MA, et al. (2008). Cerebral aspergillosis caused by Aspergillus ustus following orthotopic heart transplantation: case report and review of the literature. Clinical Transplantion 23: 116–120 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Skoube P, Samson RA. (2005). Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Systematic and Applied Microbiology 28: 442–453 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U. (1987). Standardized high performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone retention indices and UV-VIS spectra (diode array detection). Journal of Chromatography 404: 195–214 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U. (1993). Liquid chromatography of mycotoxins. Journal of Chromatography Library 54: 253–372 [Google Scholar]
- Gams W, Christensen M, Onions AH, Pitt JI, Samson RA. (1985). Infrageneric taxa of Aspergillus. In: Advances in Penicillium and Aspergillus Systematics. (Samson RA, Pitt JI, eds). New York: Plenum Press: 55–62 [Google Scholar]
- Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. (2008). Isolation, structure elucidation, and biomimetic total synthesis of versicolamide B, and the isolation of antipodal (-)-stephacidin and (+)-notoamide B from Aspergillus versicolor NRRL 35600. Angewandte Chemie 47: 3573–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageskal G, Kristensen R, Fristad RF, Skaar I. (2011). Emerging pathogen Aspergillus calidoustus colonizes water distribution systems. Medical Mycology (in press). DOI: 10.3109/13693786.2010.549155 [DOI] [PubMed]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. (2011) The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis DM, Bull JJ. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192 [Google Scholar]
- Hosoe T, Sameshima T, Dobashi K, Kawai KI. (1998). Structures of two new 18,22-cyclosterols, emesterones A and B, from Emericella heterothallica. Chemical and Pharmaceutical Bulletin 46: 850–852 [Google Scholar]
- Houbraken J, Due M, Varga J, Meijer M, Frisvad JC, Samson RA. (2007). Polyphasic taxonomy of Aspergillus section Usti. Studies in Mycology 59: 107–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SS, Dorr TE, Biberstein EL, Wong A. (1986). Aspergillus deflectus infection in four dogs. Journal of Medical and Veterinary Mycology 24: 95–104 [PubMed] [Google Scholar]
- Kahler JS, Leach MW, Jang S, Wong A. (1990). Disseminated aspergillosis attributable to Aspergillus deflectus in a springer spaniel. Journal of the American Veterinary Medical Association 197: 871–874 [PubMed] [Google Scholar]
- Kawahara N, Nakajima S, Yamazaki M, Kawai K. (1989). Structure of a novel epidithiodioxopiperazine, emethallicin A, a potent inhibitor of histamine release from Emericella heterothallica. Chemical and Pharmaceutical Bulletin 37: 2592–2595 [DOI] [PubMed] [Google Scholar]
- Kawahara N, Nozawa K, Nakajima S, Kawai K. (1988). Emeheterone, a pyrazinone derivative from Emericella heterothallica. Phytochemistry 27: 3022–3024 [Google Scholar]
- Kawahara N, Nozawa K, Yamazaki M, Nakajima S, Kawai K. (1990b). Structures of novel epipolythiodioxopiperazines, emethallicins B, C, and D, potent inhibitors of histamine release, from Emericella heterothallica. Chemical and Pharmaceutical Bulletin 38: 73–78 [DOI] [PubMed] [Google Scholar]
- Kawahara N, Nozawa K, Yamazaki M, Nakajima S, Kawai KI. (1990a). Novel epidithiodioxopiperazines, emethallicins E and F, from Emericella heterothallica. Heterocycles 30: 507–515 [Google Scholar]
- Klich MA. (1993). Morphological studies of Aspergillus section Versicolores and related species. Mycologia 85: 100–107 [Google Scholar]
- Kozakiewicz Z. (1989). Aspergillus species in stored products. Mycological Papers 161: 1–188 [Google Scholar]
- Krishnan-Natesan S, Chandrasekar PH, Manavathu EK, Revankar SG. (2008). Successful treatment of primary cutaneous Aspergillus ustus infection with surgical debridement and a combination of voriconazole and terbinafine. Diagnostic Microbiology and Infectious Disease 62: 443–446 [DOI] [PubMed] [Google Scholar]
- Krockenberger MB, Swinney G, Martin P, Rothwell TR, Malik R. (2011). Sequential opportunistic infections in two German Shepherd dogs. Australian Veterinary Journal 89: 9–14 [DOI] [PubMed] [Google Scholar]
- Locquin-Linard M. (1990). Fennellia monodii, nouvelle espece d'Ascomycete coprophile de zones arides Africaines. Mycotaxon 39: 9–15 (in French) [Google Scholar]
- Mizuno R, Kawahara N, Nozawa K, Yamazaki M, Nakajima S, Kawai K. (1995). Stereochemistry of an 18,22-cyclosterol, Mer-NF8054X, from Emericella heterothallica and Aspergillus ustus. Chemical and Pharmaceutical Bulletin 43: 9–11 [Google Scholar]
- Nakai K, Kanda Y, Mineishi S, Hori A, Chizuka A, et al. (2002). Primary cutaneous aspergillosis caused by Aspergillus ustus following reduced-intensity stem cell transplantation. Annals of Hematology 81: 593–596 [DOI] [PubMed] [Google Scholar]
- Panackal AA, Imhof A, Hanley EW, Marr KA. (2006). Aspergillus ustus infections among transplant recipients. Emerging Infectious Diseases 12: 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko VT. (1964). Some new species of fungi on starch from the Ukraine. Mycologia 56: 58–63 [Google Scholar]
- Pavie J, Lacroix C, Hermoso DG, Robin M, Ferry C, et al. (2005). Breakthrough disseminated Aspergillus ustus infection in allogeneic hematopoietic stem cell transplant recipients receiving voriconazole or caspofungin prophylaxis. Journal of Clinical Microbiology 43: 4902–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peláez T, Padilla C, Gama B, Escribano P, Reigadas E, et al. (2010). Antifungal susceptibility and clinical significance of Aspergillus section Usti in a Spanish hospital. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 12–15, 2010, Boston, Massachusetts, poster No. M-376 [Google Scholar]
- Peterson SW. (2000). Phylogenetic relationships in Aspergillus based on rDNA sequence analysis. In: Integration of modern taxonomic methods for Penicillium and Aspergillus classification. (Samson RA, Pitt JI, eds.) Harwood Academic Publishers, Amsterdam: 323–355 [Google Scholar]
- Peterson SW. (2008). Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100: 205–226 [DOI] [PubMed] [Google Scholar]
- Raper KB, Fennell DI. (1965). The genus Aspergillus. Baltimore: Williams & Wilkins; [Google Scholar]
- Robinson WF, Connole MD, King TJ, Pitt JI, Moss SM. (2000). Systemic mycosis due to Aspergillus deflectus in a dog. Australian Veterinary Journal 78: 600–602 [DOI] [PubMed] [Google Scholar]
- Samson RA. (1979). A compilation of the Aspergilli described since 1965. Studies in Mycology 18: 1–38 [Google Scholar]
- Samson RA, Hoekstra ES, Frisvad JC. (eds). (2004). Introduction to food and airborne fungi 7th ed. Utrecht: Centraal Bureau voor Schimmelcultures; [Google Scholar]
- Samson RA, Mouchacca J. (1975). Additional notes on species of Aspergillus, Eurotium and Emericella from Egyptian desert soil. Antonie van Leeuwenhoek 41: 343–451 [DOI] [PubMed] [Google Scholar]
- Schultz RM, Johnson EG, Wisner ER, Brown NA, Byrne BA, Sykes JE. (2008). Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. Journal of Veterinary Internal Medicine 22: 851–859 [DOI] [PubMed] [Google Scholar]
- Slack GJ, Puniani E, Frisvad JC, Samson RA, Miller JD. (2009). Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycological Research 113: 480–490 [DOI] [PubMed] [Google Scholar]
- Smedsgaard J. (1997). Micro-scale extraction procedure for standardized screening of fungla metabolite production in cultures. Journal of Chromatography A 760: 264–270 [DOI] [PubMed] [Google Scholar]
- Steyn PS. (1971). Austamide, a new toxic metabolite from Aspergillus ustus. Tetrahedron Letters 1971: 3331–3334 [Google Scholar]
- Steyn PS. (1973). The structures of five diketopiperazines from Aspergillus ustus. Tetrahedron 29: 107–120 [Google Scholar]
- Steyn PS, Vleggaar R. (1974). Austocystins. Six novel dihydrofuro (3',2':4,5) furo(3,2-b)xanthenones from Aspergillus ustus. Journal of the Chemical Society Perkin Transactions 1 1974: 2250–2256 [PubMed] [Google Scholar]
- Steyn PS, Vleggaar R. (1975). Dihydrofuro[3',2'-4,5]furo[3,2-B]xanthones - structures of austocystin G, austocystin H and austocystin I. Journal of the South African Chemical Institute 28: 375-377 [Google Scholar]
- Steyn PS, Vleggaar R. (1976a). 12,13-Dihydro-12-hydroxyaustamide, a new dioxopiperazine from Aspergillus ustus. Phytochemistry 15: 355–356 [Google Scholar]
- Steyn PS, Vleggaar R. (1976b). The structure of dihydrodeoxy-8-epi-austdiol and the absolute configuration of the azaphilones. Journal of the Chemical Society Perkin Transactions 1 1976: 204–206 [PubMed] [Google Scholar]
- Stiller MJ, Teperman L, Rosenthal SA, Riordan A, Potter J, et al. (1994). Primary cutaneous infection by Aspergillus ustus in a 62-year-old liver transplant recipient. Journal of the American Academy of Dermatology 31: 344–347 [DOI] [PubMed] [Google Scholar]
- Swofford T. (2000). PAUP*: Phylogenetic analysis using parsimony. v. 4.0. Sinauer Associates, Sunderland: [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software v. 4.0. Molecular Biology and Evolution 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Vagefi PA, Cosimi AB, Ginns LC, Kotton CN. (2008). Cutaneous Aspergillus ustus in a lung transplant recipient: emergence of a new opportunistic fungal pathogen. Journal of Heart & Lung Transplantation 27: 131–134 [DOI] [PubMed] [Google Scholar]
- Varga J, Frisvad JC, Samson RA. (2007). Polyphasic taxonomy of Aspergillus section Candidi based on molecular, morphological and physiological data. Studies in Mycology 59: 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Frisvad JC, Samson RA. (2009). A reappraisal of fungi producing aflatoxins. World Mycotoxin Journal 2: 263–277 [Google Scholar]
- Varga J, Houbraken J, Van Der Lee HA, Verweij PE, Samson RA. (2008). Aspergillus calidoustus sp. nov., causative agent of human infections previously assigned to Aspergillus ustus. Eukaryotic Cell 7: 630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij PE, Bergh MF van den, Rath PM, Pauw BE de, Voss A, Meis JF. (1999). Invasive aspergillosis caused by Aspergillus ustus: case report and review. Journal of Clinical Microbiology 37: 1606–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleggaar R, Steyn PS, Nagel DW. (1974). Constitution and absolute configuration of austdiol, the main toxic metabolite from Aspergillus ustus. Journal of the Chemical Society Perkin Transactions 1 1974: 45–49 [DOI] [PubMed] [Google Scholar]
- Weiss LM, Thiemke WA. (1983). Disseminated Aspergillus ustus infection following cardiac surgery. American Journal of Clinical Pathology 80: 408–411 [DOI] [PubMed] [Google Scholar]
- Yabe K, Nakamura Y, Nakajima H, Ando Y, Hamasaki T. (1991). Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Applied and Environmental Microbiology 57: 1340–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiran ST, Mutlu FM, Saracli MA, Uysal Y, Gonlum A, et al. (2006). Fungal endophthalmitis caused by Aspergillus ustus in a patient following cataract surgery. Medical Mycology 44: 665–669 [DOI] [PubMed] [Google Scholar]
- Zohri AA, Ismail MA. (1994). Based on biochemical and physiological behavior, where is Aspergillus egyptiacus better placed? Folia Microbiologica 39: 415–419 [DOI] [PubMed] [Google Scholar]