Abstract
Nicotinic acetylcholine receptors are ligand-gated ion conducting transmembrane channels from the Cys-loop receptor super-family. The α4β2 subtype is the predominant heteromeric subtype of nicotinic receptors found in the brain. Allosteric modulators for α4β2 receptors interact at a site other than the orthosteric site where acetylcholine binds. Many compounds which act as allosteric modulators of the α4β2 receptors have been identified, with both positive and negative effects. Such allosteric modulators either increase or decrease the response induced by agonist on the α4β2 receptors. Here we discuss the concept of allosterism as it pertains to the α4β2 receptors and summarize the important features of allosteric modulators for this nicotinic receptor subtype.
Keywords: Nicotinic acetylcholine receptor, Allosteric modulation, Positive allosteric modulators, Negative allosteric modulators
1. Introduction
Nicotinic acetylcholine receptors (nAChRs) are cation-conducting pentameric transmembrane proteins that are activated by the endogenous neurotransmitter acetylcholine (ACh) [1]. These receptors are formed by the assembly of a variety of the 17 different subunits (α1-α10, β1- β4, γ, δ, ε) thus far known to exist, resulting in different subtypes of functional nAChRs [2]. The nAChRs at the neuromuscular junctions are composed of α1, β1, γ, δ, ε subunits and referred to as the muscle type of nAChRs, while those present at the synapse or other tissues in the nervous system are composed of α (2–10) and β (2–4) subunits and referred to as the neuronal type of nAChRs [3, 4]. The main subtypes of nAChRs widely expressed in the brain are the homomeric α7 and the heteromeric α4β2 receptors [5–10]. The α4β2 receptors are expressed in two different stoichiometries, either the (α4)2(β2)3 stoichiometry which binds to ACh and nicotine with high affinity, or the (α4)3(β2)2 stoichiometry which binds to ACh and nicotine with low affinity [11, 12]. The α4β2 receptors have been implicated in a number of neurological conditions including (but not limited to) nicotine addiction [13], Alzheimer’s disease [14], depression [15], impaired cognitive functions [16] and autism [17]. Positive allosteric modulators (PAMs) are rapidly being developed for neuronal nAChRs and they represent a promising new approach for treating disorders involving the α4β2 receptors [18–21].
1.1. Allosteric modulation of the α4β2 receptors
According to the allosteric theory, the α4β2 receptor protein has multiple conformations (states) [22]. The binding of ligand to the receptor reduces the free energy of the stabilized conformation and the energy from this ligand binding interaction provides the energy required to stabilize a certain conformation of the receptor [23]. Accordingly, an agonist for the α4β2 receptor is a ligand which stabilizes the open ion conducting conformation of the receptor. The binding site of ACh occurs at the α4(+)/β2(−) interface between the subunits forming the α4β2 receptor. For the purposes of this review, we have defined the ACh binding site on the α4β2 receptor as the orthosteric site. In addition to the orthosteric site, multiple distinct binding sites on the α4β2 receptor can be present where different ligands can bind. All such sites other than the orthosteric site are defined as allosteric sites. This is the situation in the related GABAA receptor which has multiple allosteric sites [24–26]. If an allosteric ligand stabilizes the open ion conducting conformation of the receptor channel that is induced by the agonist, such ligands are termed positive allosteric modulators (PAMs) for the receptor [18, 27, 28].
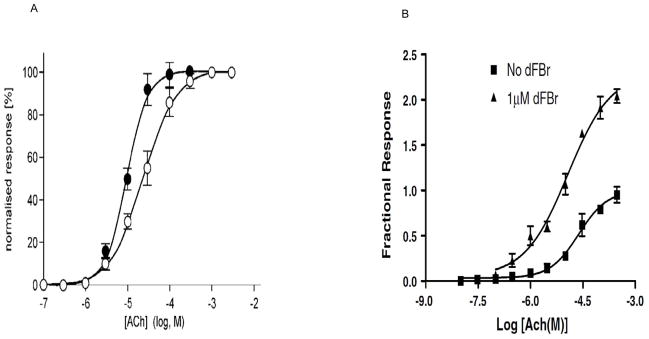
On a macroscopic level for purposes of this review, we will define PAMs of the α4β2 receptors as ligands that increase potency (i.e. an increase in the apparent affinity; Fig. 1A & B) by acting at a site different from the orthosteric site [28]. PAMs for the α4β2 receptor can also increase efficacy (maximal response amplitude), however based on our working definition here, this is not a requirement for a PAM [28, 29]. The change in efficacy caused by PAMs is rather varied and is discussed in section 1.2. On a single channel level the effect of PAMs for α4β2 receptors translates into an increase in the number of openings or an increase in mean open times of the channel, indicating the stabilization of the open state of the receptor [18].
Fig. 1.
Effect of α4β2 nAChRs PAMs, galantamine and dFBr on efficacy. A) Galantamine does not change the maximal response obtained by Ach in α4β2 nAChR stably expressed in cultured HEK-293 cells [29]. B) dFBr increases the maximal response obtained by Ach in α4β2 nAChR expressed in Xenopus oocytes [28].
Some PAMs, in addition to being allosteric modulators by acting at an allosteric binding site, also act as partial or full agonists for the α4β2 receptor [20]. If such compounds are applied along with an agonist, then an increase in receptor response amplitude is observed over what would normally be achieved by the agonist alone. This is seen with some of the 2-amino-5-ketothiazole compounds when tested on the α4β2 receptors [20]. Such ligands may be classified as allosteric agonists or allosteric partial agonists; however for the purposes of this review, we do not consider these to be pure PAMs. As we have defined here, a pure PAM is one which binds to an allosteric site on the receptor, but fails to activate the channel by itself.
Negative allosteric modulators (NAMs) are ligands which decrease the maximal current (efficacy) or affinity of the agonist, and many NAMs have been discovered for the α4β2 receptor [30–35]. Similar to the PAMs, the NAMs bind to a site on the α4β2 receptors other than the orthosteric site, and inhibit receptor function by preferentially favoring a non-conducting conformation of the receptor. NAMs for the α4β2 receptor may bind in the lumen of the channel or at non-luminal sites on the receptor such as the extracellular-transmembrane interface, the intracellular loop or anywhere else on the protein [32, 33]. There are several compounds for α4β2 receptors that are referred to as allosteric antagonists or noncompetitive antagonists [32, 33, 35–37], all of which would be considered to be NAMs as long as they bind to a site distinct from the orthosteric site and inhibit the function of the α4β2 receptors.
There may also be compounds yet to be discovered for α4β2 receptors which could potentially bind to the same allosteric site as a PAM on the receptor and competitively inhibit its binding and actions. For example for the GABAA receptors, one such compound is Ro15-1788 (Flumazenil) which binds to the benzodiazepine site and acts as an allosteric antagonist [25, 38, 39]. Such ligands for the α4β2 receptor, in particular or for other subtypes of nAChR, in general, may be consequential as they can inhibit the potentiation induced by PAMs by competing for the same allosteric binding site.
1.2. Efficacy changes in α4β2 receptors by PAMs
The effect of PAMs on potentiating the maximal response amplitudes of α4β2 receptors is varied. While a PAM increases the apparent affinity (potency) of the agonist for the α4β2 receptors, it may or may not change the maximal response amplitude (efficacy) elicited. Galantamine and desformylflustrabromine (dFBr) are both PAMs for the α4β2 receptors and display different effects on receptor efficacy. Galantamine when co-applied with ACh on human α4β2 nAChRs stably expressed in cultured HEK-293 cells causes an increase in apparent affinity without any change in efficacy (Fig. 1A) [29]. In contrast, dFBr (when co-applied with ACh) increases both apparent affinity and efficacy (Fig. 1B) [28]. This later effect is similar to that is seen with PNU-120596 on α7 receptors [40]. In the related GABAA receptor, chlordiazepoxide, a PAM acting via the benzodiazepine site, shifts the GABA dose-response curve to the left (i.e. increased potency), but does not increase the maximal GABA response amplitude [41]. However, another drug tracazolate, a pyrazolopyridine derivative which is structurally diverse from benzodiazepines, acts via the benzodiazepine site to increases both potency and efficacy [42]. A similar situation may exist in the α4β2 nAChRs.
Based on their effect on the receptor response profiles, PAMs for the α7 nAChRs have been classified into two types [19]; type I PAMs predominantly affect the peak current of the response, while type II PAMs affect the peak response and the time course of the agonist-evoked response [19]. While this classification may adequately describe the different types of PAMs for α7 receptors, this may not be the case with α4β2 receptor PAMs.
2. Positive allosteric modulators of α4β2 subtype of nAChRs
Many ligands are known to act as allosteric modulators for the α4β2 subtypes of nAChRs. Our knowledge of specific domains crucial to the binding of PAMs and the functional effects produced by them is currently incomplete. In the section that follows, the different allosteric ligands for the α4β2 receptors are discussed.
2.1 Steroids
The primary mechanistic action of steroid hormones derived from cholesterol is to control gene transcription through activation of nuclear receptors. However they also directly influence the function of a variety of ligand-gated ion channels. It has been demonstrated that albumin-bound and unbound progesterone are equally capable of inhibiting α4-subunit containing receptor activity in Xenopus oocytes [34]. Since albumin-bound progesterone cannot cross the cell membrane, an extracellular site of action for progesterone on the α4 subunit was suggested [34]. Progesterone was tested for its ability to potentiate α4β2 receptors, but it failed to do so, indicating that it can be considered a NAM.
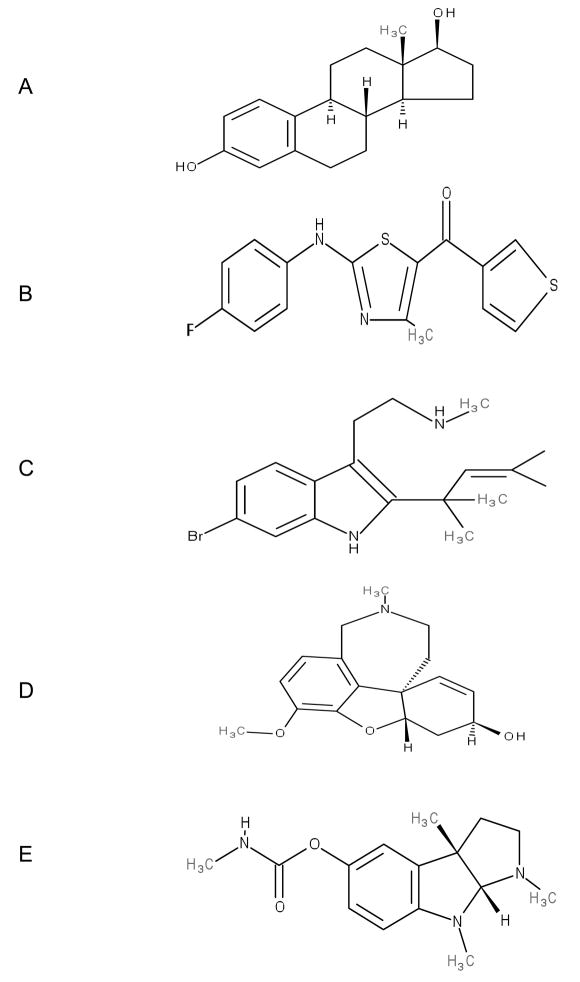
The estrogenic steroid 17-β-estradiol (17-BE) (Fig. 2A) has differential effects on α4β2 receptors. It potentiated the human but inhibited the rat receptors without potentiation [43]. This difference in action of 17-BE in these two species of α4β2 receptors was used to locate the binding site on the human receptor responsible for potentiation. Using site directed mutagenesis, it was determined that the short C-terminal end of the α4 subunit with the amino acid sequence AGMI was the key determinant for 17-BE potentiation of human α4β2 receptors (Fig. 3B) and this site was shown to be different than the steroid inhibition site [44]. Furthermore it seems as if the binding site for 17-BE is portable since it can be moved to the C-terminus of the β2 subunit and to another location on a subunit that contributes to an ACh-binding site [45]. Therefore the complex modulation of α4β2 receptors by steroids seems to be determined by both the chemical structure of the steroid and the amino acid sequence of the nAChR.
Fig. 2.
Structure of PAMs for α4β2 nAChRs. A) 17-β-estradiol, B) LY-2087101, C) Desformylflustrabromine, D) Galantamine, E) Physostigmine.
Fig. 3.
A. Location of Zn2+ sites in relation to the ACh binding sites on the (α4)3(β2)2 stoichiometry of the α4β2 nAChRs. The α4(+)/α4(−) interface influences the potentiation of (α4)3(β2)2 receptors by Zn2+. The α4(−)/β2(+) interface present in both stoichiometry of α4β2 receptors is responsible for the inhibitory action of Zn2+ [51]. B) Location of the 17-BE binding site on the C-terminal region of α4-subunit of the α4β2 nAChRs [44, 45].
There is evidence suggesting a transmembrane location of an inhibitory binding site for different steroids in other subtypes of nAChRs [33], however such experiments are yet to be done for the α4β2 receptors. Single channel studies have confirmed that mechanistically, 17-BE increases the open probability of α4β2 receptors [46] and that it appears to be a pure PAM for this subtype of nAChR.
2.2 Zinc
Ionic zinc (Zn2+) is a key modulator of neuronal excitability. It is present in neurons throughout the brain, especially in the cerebral cortex and the hippocampus [47]. It also modulates the function of many ligand-gated ion channel receptors including GABAA [48] and glycine receptors [49]. For many of the different nAChR subtypes, Zn2+can both potentiate and/or inhibit receptor function, depending on a variety of factors [50]. For the α4β2 receptor, the half-maximal concentration of Zn2+ for potentiation of ACh responses (pEC50) was about 16 μM and the half-maximal concentration for inhibition (IC50) was about 440 μM [50]. The ACh response amplitudes on α4β2 receptors were potentiated by ~ 260% with Zn2+. Furthermore the potentiation and inhibition of these receptors by Zn2+ is pH dependent, but voltage independent [51].
Of the two different stoichiometries of the α4β2 receptors found in the central nervous system, Zn2+ potentiates only the low ACh affinity stoichiometry receptors [i.e. (α4)3(β2)2] while inhibiting both high [i.e. (α4)2(β2)3] and low affinity stoichiometries of the receptor [51]. The inhibition of Zn2+ on the high affinity (α4)2(β2)3 receptor is voltage-dependent, while it is voltage-independent on low affinity (α4)3(β2)2 receptors [51]. Based on these observations and through site-directed mutagenesis studies, the β2(+)/α4(−) subunit interface was proposed as the Zn2+ inhibition site on the high and low affinity α4β2 receptors and the α4(+)/α4(−) subunit interface was proposed as the site for the potentiating action of Zn2+ on low affinity receptors (Fig. 3A) [51]. Single channel studies suggest that Zn2+ potentiates α4β4 receptors by increasing the burst frequency of the receptor [52]. Since Zn2+ potentiates α4β4 and α4β2 receptors, the mechanism of action of Zn2+ on α4β2 receptors may be similar to its mechanism of action on α4β4 receptors. Finally, Zn2+ can be considered to be a pure PAM for α4β2 receptors since it does not show any agonistic actions.
2.3 Thiazole (2-amino-5-keto) and Carbamate Analogues
Three novel (2-amino-5-keto) thiazole analogues (e.g. LY-2087101) (Fig. 2B) have been reported to be allosteric modulators of neuronal nAChRs [20]. These compounds potentiated some, but not all of the various subtypes of nAChRs, including α4β2 receptors [20]. All three compounds enhanced the potency and maximal efficacy of different nAChR agonists on α4β2 receptors, a profile typical of allosteric potentiators [20]. At concentrations required for potentiation, the compounds did not displace [3H]-epibatidine from the agonist-binding site and potentiation was observed at all agonist concentrations, suggesting a non-competitive allosteric mechanism of action [20]. Interestingly at concentrations higher than that required for potentiation, these compounds also showed intrinsic agonist activity which was blocked by competitive and non-competitive nAChR antagonists [20]. Thus these compounds are not pure PAMs for α4β2 receptors.
Recently a series of carbamate analogues which were optimized for CNS penetration were reported to potentiate α4β2 nAChRs [53]. These compounds increased the response of α4β2 receptors to ACh [54] without competing for binding with the orthosteric ligand cytisine [53]. These carbamate analogues represent a new class of compounds with immense therapeutic potential.
2.4 Desformylflustrabromine (dFBr)
Desformylflustrabromine (dFBr) (Fig. 2C) is a metabolite of the marine bryozoan Flustra foliacea which is common in the North Sea [55]. Some of the tryptophan-derived metabolites of Flustra were first shown to have muscle relaxant properties [56]. Flustramine A, another Flustra extract has also been shown to block potassium channels [57]. When the Flustra derivatives were tested on the α4β2 and α7 nAChR subtypes using radioligand binding assays, it was determined that dFBr had low μM affinities for these receptors [58]. dFBr extracted from its natural source was tested functionally on various subtypes of nAChRs, including α4β2, expressed in Xenopus oocytes [18]. When co-applied with ACh, dFBr selectively potentiated but did not inhibit α4β2 receptors. By using synthetic dFBr, it was later shown that the ACh-induced responses of α4β2 receptor were potentiated in the nano-molar range (pEC50 = 120 nM) and inhibited in the micro-molar range (IC50= 150 μM) [28]. This potentiation induced by dFBr was reversible and concentration dependent. Furthermore in single channel recordings, dFBr acts by either increasing the opening rate constant or decreasing the closing rate constant of the α4β2 receptors, without changing the conductance or reversal potential [18]. It has been proposed that dFBr potentiates α4β2 receptors by an allosteric mechanism while its inhibition is via an open channel block [27]. Recently we have shown that in addition to enhancing the current amplitude of both α4β2 and α2β2 nAChRs, dFBr also prevents the inhibition of these channels by the β-amyloid peptide (Aβ1-42), a result that suggests that similar compounds may be useful in combating the inhibition of these receptors by Aβ1-42 in patients suffering with Alzheimer’s Disease [59]. There is no evidence of dFBr acting as an agonist on any subtype of nAChRs.
2.5 Acetylcholinesterase inhibitors
Acetylcholinesterase (AChE) is the enzyme that rapidly hydrolyzes ACh. Some AChE inhibitors such as galantamine (Fig. 2D) and physostigmine (Fig. 2E) also act as noncompetitive allosteric agonists on nAChRs. Recent reports based on homology models for the ligand binding domain of human α7 and α4β2 receptors have identified the key amino acid residues which are presumably, an important part of the binding site for these compounds [60]. This binding site region overlaps the ACh binding site on nAChR and is located on the outer surface of the ligand binding domain. This suggests that galantamine, physostigmine and ACh bind to different sites on nAChR and that allosteric potentiation may arise from a direct interplay between both these sites [60].
Physostigmine and galantamine have effects on α4β2 receptors that are similar to their action on muscle nicotinic receptors [61]. Galantamine shows no effect on α4β2 receptors expressed in Xenopus oocytes. In contrast however, in HEK293 cells expressing human α4β2 receptors, galantamine reduces the apparent EC50 of ACh by half but has no effect on the efficacy [29]. Like galantamine, physostigmine also shows differential effects on receptor function depending on the expression system. For example physostigmine activates chicken α4β2 receptors expressed in M10 cells through a binding sight that is insensitive to ACh [61], however it does not elicit currents from α4β2 receptors when expressed in Xenopus oocytes. When physostigmine is co-applied with low concentration (1 μM) of ACh on α4β2 receptors expressed in Xenopus oocytes, it potentiates ionic currents, however it inhibits them when co-applied with higher ACh concentrations [62]. A two-site equilibrium receptor occupation model predicts that these dual effects of physostigmine are due to its binding to a second equivalent agonist recognition site combined with noncompetitive ion channel block [62, 63]. Based on this mechanism, it can be concluded that the binding site of physostigmine on α4β2 receptors is also a low affinity ACh binding site distinct from the high affinity site that activates the receptor [62].
Both physostigmine and galantamine activate single channels in excised membrane patches from cultured M10 cells expressing the neuronal α4β2 receptors [61] and both are thought to potentiate neuronal nAChRs by stabilizing the open state of the receptor channel [64]. Galantamine and physostigmine lack selectivity in their allosteric action because they potentiate both homomeric α7 and other heteromeric subtypes of nAChRs. The beneficial effects of these two drugs in restoring cholinergic tone in the synapse is expected to be enhanced by their dual actions as acetylcholinesterase inhibitors and allosteric modulators of nAChRs [65].
3. Negative allosteric modulators of α4β2 subtype of nAChRs
The α4β2 subtype of nAChRs is targeted by a number of exogenous and endogenous compounds that allosterically modulate its function [33]. Such compounds are structurally diverse and inhibit the functioning of the receptor and are referred to as negative allosteric modulators (NAMs). Compounds that are true NAMs have a distinct allosteric binding site on the α4β2 receptor, whether it is on the extracellular domain or the intracellular loop, the transmembrane domain or in the pore of the ion channel itself.
Drugs like dizocilpine (+)-MK-801, amantadine and memantine act as open channel blockers for the α4β2 receptor and have a specific binding site in the pore of the receptor channel [37], therefore they are classified as NAMs for this receptor. The antidepressant milnacipran [66] and steroids such as progesterone [34] are some of the other known NAMs for the α4β2 receptors, as is UCI-30002 [N-(1,2,3,4-tetrahydro-1-naphthyl)-4-nitroaniline] which has been shown to significantly reduce nicotine self-administration in rats [30]. KAB-18 is another α4β2 nAChR NAM that contains three phenyl rings, one piperidine ring and one ester bond linkage was developed with the aid of SAR computational designing and molecular biology approaches [31].
While NAMs for α4β2 receptors indicate the presence of an allosteric binding site on the receptor, they may not offer any distinct advantage as compared to a competitive antagonist in terms of drug development. However, NAMs can be useful experimental tools for studying the functional aspects of the α4β2 nAChR function.
4. Conclusions
Due to their importance in the regulation of neuronal signaling and their apparent role in neurological disorders, a significant effort has been made to develop nAChR ligands, particularly those that can act selectively on individual nAChR subtypes. For the α4β2 receptors, two broad types of ligands are being actively pursued. These can be classified as orthosteric ligands (ligands that bind at the ACh binding site) and allosteric ligands (ligands binding outside of the orthosteric ACh binding site). Orthosteric ligands have received much attention. Partial agonists like varenicline, which targets the α4β2 nAChR subtype, provides a treatment option for smoking cessation and nicotine addiction [67, 68]. However, most partial or full agonists cause desensitization of receptor responses [69, 70] and up regulation of receptors expression [71, 72]. Hence, the utility of either partial or full agonists can be limited. Subtype selective agonists have also proved difficult to develop, probably due to the conserved nature of the orthosteric binding sites[54]. Given the variety of allosteric binding sites that apparently exist on nAChRs, it may be easier to develop subtype selective allosteric (rather than orthosteric) ligands since allosteric sites are likely to be less conserved across nAChR subtypes. Therefore, these ligands may display greater subtype selectivity.
PAMs bind to allosteric binding sites and produce an increase in the potency of the agonist and often in the amplitude of responses [19]. Allosteric ligands that enhance the currents elicited by ACh provide an alternate approach to treating disorders of decreased nAChR activity. Pure PAMs potentiate responses in the presence of endogenous agonists and do not, by themselves, activate the receptor. This can prevent receptor desensitization and preserve cholinergic modulation of synaptic transmission. This is in contrast to orthosteric ligands like nicotine which produce receptor up-regulation and/or desensitization [69–72]. In pathological conditions where nAChR-mediated signaling in the CNS is decreased, PAMs could be used to maintain or restore normal levels of nAChR-mediated synaptic transmission without directly altering other cholinergic signaling (e.g. that through the mAChRs).
Since α4β2 receptors are implicated in the patho-physiology of many diseases and conditions, PAMs for these receptors could be important therapeutic agents in the treatment of a variety of neuro-pathological conditions. The therapeutic applicability of the benzodiazepine class of compounds, for example, is an amazing success story in the related GABA receptor [24, 26]. Allosteric modulatory ligands for nAChRs provide many advantages over conventional agonists. PAMs for the α4β2 nAChR clearly have the potential to develop into clinically applicable drugs targeting these receptors. Such compounds will provide a novel means of treatment for various neurological disorders where these receptors play a critical role.
Acknowledgments
We would like to thank and acknowledge the contributions of Patricia Lamb for her input in the preparation of this manuscript. We would also like to extend our appreciation to Dr. Christian Erxleben and Dr. Nikhil. A. Gokhale for their help in the review of the manuscript. Participants in this research review are funded and supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, NIH.
Abbreviation
- ACh
acetylcholine
- nAChRs
neuronal nicotinic acetylcholine receptors
- dFBr
desformylflustrabromine
- PAM
positive allosteric modulator
- NAM
negative allosteric modulator
- 17-BE
17-β-Estradiol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yakel JL. Gating of nicotinic ACh receptors: latest insights into ligand binding and function. J Physiol. 588:597–602. doi: 10.1113/jphysiol.2009.182691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–46. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- 4.Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–96. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–7. [PubMed] [Google Scholar]
- 6.Gopalakrishnan M, Monteggia LM, Anderson DJ, Molinari EJ, Piattoni-Kaplan M, Donnelly-Roberts D, et al. Stable expression, pharmacologic properties and regulation of the human neuronal nicotinic acetylcholine alpha 4 beta 2 receptor. J Pharmacol Exp Ther. 1996;276:289–97. [PubMed] [Google Scholar]
- 7.Lindstrom J. Neuronal nicotinic acetylcholine receptors. Ion Channels. 1996;4:377–450. doi: 10.1007/978-1-4899-1775-1_10. [DOI] [PubMed] [Google Scholar]
- 8.Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1996;109:125–37. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, et al. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–29. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiting PJ, Schoepfer R, Swanson LW, Simmons DM, Lindstrom JM. Functional acetylcholine receptor in PC12 cells reacts with a monoclonal antibody to brain nicotinic receptors. Nature. 1987;327:515–8. doi: 10.1038/327515a0. [DOI] [PubMed] [Google Scholar]
- 11.Khiroug SS, Khiroug L, Yakel JL. Rat nicotinic acetylcholine receptor alpha2beta2 channels: comparison of functional properties with alpha4beta2 channels in Xenopus oocytes. Neuroscience. 2004;124:817–22. doi: 10.1016/j.neuroscience.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–31. [PubMed] [Google Scholar]
- 13.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 14.Lamb PW, Melton MA, Yakel JL. Inhibition of neuronal nicotinic acetylcholine receptor channels expressed in Xenopus oocytes by beta-amyloid1-42 peptide. J Mol Neurosci. 2005;27:13–21. doi: 10.1385/JMN:27:1:013. [DOI] [PubMed] [Google Scholar]
- 15.Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, et al. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline's effect. Eur J Pharmacol. 2009;605:114–6. doi: 10.1016/j.ejphar.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–30. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Ruiz CM, Lee M, Perry RH, Baumann M, Court JA, Perry EK. Molecular analysis of nicotinic receptor expression in autism. Brain Res Mol Brain Res. 2004;123:81–90. doi: 10.1016/j.molbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Sala F, Mulet J, Reddy KP, Bernal JA, Wikman P, Valor LM, et al. Potentiation of human alpha4beta2 neuronal nicotinic receptors by a Flustra foliacea metabolite. Neurosci Lett. 2005;373:144–9. doi: 10.1016/j.neulet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–63. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Broad LM, Zwart R, Pearson KH, Lee M, Wallace L, McPhie GI, et al. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J Pharmacol Exp Ther. 2006;318:1108–17. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- 21.Timmermann DB, Gronlien JH, Kohlhaas KL, Nielsen EO, Dam E, Jorgensen TD, et al. An allosteric modulator of the alpha7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- 22.Monod J, Wyman J, Changeux JP. On The Nature Of Allosteric Transitions: A Plausible Model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 23.Lena C, Changeux JP. Allosteric modulations of the nicotinic acetylcholine receptor. Trends Neurosci. 1993;16:181–6. doi: 10.1016/0166-2236(93)90150-k. [DOI] [PubMed] [Google Scholar]
- 24.Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–5. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 25.Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995;16:162–8. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- 26.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 27.Weltzin MM, Schulte MK. Pharmacological characterization of the allosteric modulator desformylflustrabromine and its interaction with alpha4beta2 neuronal nicotinic acetylcholine receptor orthosteric ligands. J Pharmacol Exp Ther. 2010;334:917–26. doi: 10.1124/jpet.110.167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JS, Padnya A, Weltzin M, Edmonds BW, Schulte MK, Glennon RA. Synthesis of desformylflustrabromine and its evaluation as an alpha4beta2 and alpha7 nACh receptor modulator. Bioorg Med Chem Lett. 2007;17:4855–60. doi: 10.1016/j.bmcl.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–36. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura RF, Hogenkamp DJ, Li WY, Tran MB, Belluzzi JD, Whittemore ER, et al. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self-administration in rats. J Pharmacol Exp Ther. 2007;323:907–15. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]
- 31.Henderson BJ, Pavlovicz RE, Allen JD, Gonzalez-Cestari TF, Orac CM, Bonnell AB, et al. Negative allosteric modulators that target human alpha4beta2 neuronal nicotinic receptors. J Pharmacol Exp Ther. 334:761–74. doi: 10.1124/jpet.110.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arias HR, Bhumireddy P, Bouzat C. Molecular mechanisms and binding site locations for noncompetitive antagonists of nicotinic acetylcholine receptors. Int J Biochem Cell Biol. 2006;38:1254–76. doi: 10.1016/j.biocel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Arias HR. Binding sites for exogenous and endogenous non-competitive inhibitors of the nicotinic acetylcholine receptor. Biochim Biophys Acta. 1998;1376:173–220. doi: 10.1016/s0304-4157(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 34.Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89:9949–53. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arias HR. Positive and negative modulation of nicotinic receptors. Adv Protein Chem Struct Biol. 80:153–203. doi: 10.1016/B978-0-12-381264-3.00005-9. [DOI] [PubMed] [Google Scholar]
- 36.Arias HR. Biological and biophysical aspects of ligand-gated ion channel receptor superfamilies, 2006. Research Signpost; Kerala, India: 2006. [Google Scholar]
- 37.Buisson B, Bertrand D. Open-channel blockers at the human alpha4beta2 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:555–63. doi: 10.1124/mol.53.3.555. [DOI] [PubMed] [Google Scholar]
- 38.Bormann J. Electrophysiological characterization of diazepam binding inhibitor (DBI) on GABAA receptors. Neuropharmacology. 1991;30:1387–9. doi: 10.1016/s0028-3908(11)80006-7. [DOI] [PubMed] [Google Scholar]
- 39.Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–9. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- 40.Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maksay G, Thompson SA, Wafford KA. Allosteric modulators affect the efficacy of partial agonists for recombinant GABA(A) receptors. Br J Pharmacol. 2000;129:1794–800. doi: 10.1038/sj.bjp.0703259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheleznova N, Sedelnikova A, Weiss DS. alpha1beta2delta, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Br J Pharmacol. 2008;153:1062–71. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paradiso K, Sabey K, Evers AS, Zorumski CF, Covey DF, Steinbach JH. Steroid inhibition of rat neuronal nicotinic alpha4beta2 receptors expressed in HEK 293 cells. Mol Pharmacol. 2000;58:341–51. doi: 10.1124/mol.58.2.341. [DOI] [PubMed] [Google Scholar]
- 44.Paradiso K, Zhang J, Steinbach JH. The C terminus of the human nicotinic alpha4beta2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci. 2001;21:6561–8. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin X, Steinbach JH. A Portable Site: A Binding Element for 17{beta}-Estradiol Can Be Placed on Any Subunit of a Nicotinic {alpha}4{beta}2 Receptor. J Neurosci. 31:5045–54. doi: 10.1523/JNEUROSCI.4802-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtis L, Buisson B, Bertrand S, Bertrand D. Potentiation of human alpha4beta2 neuronal nicotinic acetylcholine receptor by estradiol. Mol Pharmacol. 2002;61:127–35. doi: 10.1124/mol.61.1.127. [DOI] [PubMed] [Google Scholar]
- 47.Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130:1471S–83S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 48.Krishek BJ, Moss SJ, Smart TG. Interaction of H+ and Zn2+ on recombinant and native rat neuronal GABAA receptors. J Physiol. 1998;507 (Pt 3):639–52. doi: 10.1111/j.1469-7793.1998.639bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor alpha1 subunit. J Physiol. 1999;520(Pt 1):53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsiao B, Dweck D, Luetje CW. Subunit-dependent modulation of neuronal nicotinic receptors by zinc. J Neurosci. 2001;21:1848–56. doi: 10.1523/JNEUROSCI.21-06-01848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moroni M, Vijayan R, Carbone A, Zwart R, Biggin PC, Bermudez I. Non-agonist-binding subunit interfaces confer distinct functional signatures to the alternate stoichiometries of the alpha4beta2 nicotinic receptor: an alpha4-alpha4 interface is required for Zn2+ potentiation. J Neurosci. 2008;28:6884–94. doi: 10.1523/JNEUROSCI.1228-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsiao B, Mihalak KB, Magleby KL, Luetje CW. Zinc potentiates neuronal nicotinic receptors by increasing burst duration. J Neurophysiol. 2008;99:999–1007. doi: 10.1152/jn.01040.2007. [DOI] [PubMed] [Google Scholar]
- 53.Albrecht BK, Berry V, Boezio AA, Cao L, Clarkin K, Guo W, et al. Discovery and optimization of substituted piperidines as potent, selective, CNS-penetrant alpha4beta2 nicotinic acetylcholine receptor potentiators. Bioorg Med Chem Lett. 2008;18:5209–12. doi: 10.1016/j.bmcl.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 54.Springer SK, Woodin KS, Berry V, Boezio AA, Cao L, Clarkin K, et al. Synthesis and activity of substituted carbamates as potentiators of the alpha4beta2 nicotinic acetylcholine receptor. Bioorg Med Chem Lett. 2008;18:5643–7. doi: 10.1016/j.bmcl.2008.08.092. [DOI] [PubMed] [Google Scholar]
- 55.Lysek N, Rachor E, Lindel T. Isolation and structure elucidation of deformylflustrabromine from the North Sea bryozoan Flustra foliacea. Z Naturforsch C. 2002;57:1056–61. doi: 10.1515/znc-2002-11-1218. [DOI] [PubMed] [Google Scholar]
- 56.Sjoblom T, Bohlin L, Christophersen C. Studies of Swedish marine organisms. II. Muscle-relaxant alkaloids from the marine bryozoan Flustra foliacea. Acta Pharm Suec. 1983;20:415–8. [PubMed] [Google Scholar]
- 57.Peters L, Konig GM, Terlau H, Wright AD. Four new bromotryptamine derivatives from the marine bryozoan Flustra foliacea. J Nat Prod. 2002;65:1633–7. doi: 10.1021/np0105984. [DOI] [PubMed] [Google Scholar]
- 58.Peters L, Wright AD, Kehraus S, Gundisch D, Tilotta MC, Konig GM. Prenylated indole alkaloids from Flustra foliacea with subtype specific binding on NAChRs. Planta Med. 2004;70:883–6. doi: 10.1055/s-2004-832610. [DOI] [PubMed] [Google Scholar]
- 59.Pandya A, Yakel JL. Allosteric Modulator Desformylflustrabromine Relieves the Inhibition of alpha2beta2 and alpha4beta2 Nicotinic Acetylcholine Receptors by beta-Amyloid(1–42) Peptide. J Mol Neurosci. doi: 10.1007/s12031-011-9509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luttmann E, Ludwig J, Hoffle-Maas A, Samochocki M, Maelicke A, Fels G. Structural model for the binding sites of allosterically potentiating ligands on nicotinic acetylcholine receptors. ChemMedChem. 2009;4:1874–82. doi: 10.1002/cmdc.200900320. [DOI] [PubMed] [Google Scholar]
- 61.Pereira EF, Alkondon M, Reinhardt S, Maelicke A, Peng X, Lindstrom J, et al. Physostigmine and galanthamine: probes for a novel binding site on the alpha 4 beta 2 subtype of neuronal nicotinic acetylcholine receptors stably expressed in fibroblast cells. J Pharmacol Exp Ther. 1994;270:768–78. [PubMed] [Google Scholar]
- 62.Smulders CJ, Zwart R, Bermudez I, van Kleef RG, Groot-Kormelink PJ, Vijverberg HP. Cholinergic drugs potentiate human nicotinic alpha4beta2 acetylcholine receptors by a competitive mechanism. Eur J Pharmacol. 2005;509:97–108. doi: 10.1016/j.ejphar.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 63.Zwart R, van Kleef RG, Gotti C, Smulders CJ, Vijverberg HP. Competitive potentiation of acetylcholine effects on neuronal nicotinic receptors by acetylcholinesterase-inhibiting drugs. J Neurochem. 2000;75:2492–500. doi: 10.1046/j.1471-4159.2000.0752492.x. [DOI] [PubMed] [Google Scholar]
- 64.Maelicke A, Samochocki M, Jostock R, Fehrenbacher A, Ludwig J, Albuquerque EX, et al. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer's disease. Biol Psychiatry. 2001;49:279–88. doi: 10.1016/s0006-3223(00)01109-4. [DOI] [PubMed] [Google Scholar]
- 65.Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albuquerque EX. Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer's disease. Behav Brain Res. 2000;113:199–206. doi: 10.1016/s0166-4328(00)00214-x. [DOI] [PubMed] [Google Scholar]
- 66.Ueta K, Suzuki T, Uchida I, Mashimo T. In vitro inhibition of recombinant ligand-gated ion channels by high concentrations of milnacipran. Psychopharmacology (Berl) 2004;175:241–6. doi: 10.1007/s00213-004-1808-8. [DOI] [PubMed] [Google Scholar]
- 67.Spiller K, Xi ZX, Li X, Ashby CR, Jr, Callahan PM, Tehim A, et al. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology. 2009;57:60–6. doi: 10.1016/j.neuropharm.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 69.Buccafusco JJ, Beach JW, Terry AV., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328:364–70. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu Y, Marks MJ, Collins AC. Desensitization of nicotinic agonist-induced [3H]gamma-aminobutyric acid release from mouse brain synaptosomes is produced by subactivating concentrations of agonists. J Pharmacol Exp Ther. 1999;291:1127–34. [PubMed] [Google Scholar]
- 71.Whiteaker P, Sharples CG, Wonnacott S. Agonist-induced up-regulation of alpha4beta2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol Pharmacol. 1998;53:950–62. [PubMed] [Google Scholar]
- 72.Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]