Abstract
Purpose
To report our results of nephron-sparing radiofrequency ablation (RFA) of renal tumors.
Materials and Methods
Since August 2004, 49 patients with renal tumors were treated with either percutaneous or laparoscopic RFA. All patients underwent preoperative imaging with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and were suspected to have renal cell carcinoma. The follow-up for each patient included a physical examination, chest radiography, liver function tests, and a contrast-enhanced CT or MRI. To confirm the pathologic criteria of complete ablation, 30 patients underwent 6-month or 1-year follow-up biopsy. Recurrence was defined as growth of the tumor or any new enhancing portions at 3 months after confirmed nonenhancement of the initial RFA lesion.
Results
Technical success was achieved in 46/49 cases (94%). The mean tumor size was 2.4 cm and the mean follow-up period was 31.7 months (range, 6-68 months). Of 49 patients, repeated RFA was necessary in 7 patients (14%). Three patients were found to have recurrence at various follow-up intervals. Twenty-three patients (47%) experienced complications, and all but one necessitated intervention. No distant metastasis was found in any cases, and all patients are alive and are being serially followed up.
Conclusions
Percutaneous or laparoscopic RFA is considered to be a useful treatment for selected patients with small renal masses and for nephron-sparing. With a mean follow-up of 31.7 months, our intermediate data suggest excellent therapeutic outcome with RFA with effective local tumor control and preservation of renal function. The ultimate role of this modality will continue to evolve and warrants further studies.
Keywords: Catheter ablation, Kidney neoplasms, Minimally invasive surgical procedures
INTRODUCTION
The recent advancement and widespread use of noninvasive abdominal imaging modalities have contributed to increasing the detection of small renal masses (SRMs) [1-3]. Incidentally discovered SRMs are typically lowstage, slow-growing masses and are almost uniformly confined to the kidney at the initial diagnosis [4]. The natural history of incidentally discovered SRMs remains unclear. However, 70% to 80% are reported to be renal cell carcinomas (RCCs) when excised [5-7].
Standard treatment of localized RCC has been by either open or laparoscopic radical nephrectomy (RN). However, it has been reported that a significant number of patients who have only a single kidney after RN are at increased risk of developing chronic kidney disease (CKD) [5,8]. Lucas et al reported that RN carries seven times the risk of developing stage 3 CKD as in similar patients undergoing partial nephrectomy or radiofrequency ablation (RFA) [5]. Also, RN may certainly be considered over-treatment of many of these SRMs.
Recent advances in surgical techniques have introduced the use of nephron-sparing surgery such as open and laparoscopic partial nephrectomy, which have been shown to confer equivalent oncologic and functional outcomes to those of RN for patients with renal tumors smaller than 4 cm [9-12]. However, nephron-sparing surgery is a technically challenging procedure and is also related to increased perioperative complications and patient morbidity [11]. This is particularly true for laparoscopic partial nephrectomy, which requires advanced laparoscopic skills as well as dexterous intracorporeal suturing.
Recently, several energy-ablative technologies have been actively investigated, and thermal ablative techniques represent the newest frontier for active treatment of SRMs. The potential benefits of ablative approaches over extirpative techniques include reduced perioperative morbidity, shorter hospital stay, faster recovery, and preservation of renal function [13]. The most attractive merit of the ablative technique would be to offer nephron-sparing treatment to patients who are otherwise poor surgical candidates. Also, thermal ablation represents a paradigm shift in the management strategies of malignant tumors. Here, malignant tissue is destroyed in situ rather than being surgically removed. Surgical margin status is not considered as a treatment endpoint, thus further underscoring that principles defining successful ablation are different than those for surgical extirpation.
Radiofrequency ablation is a minimally invasive treatment that was initially used for liver tumors. In 1997 Zlotta et al first reported RFA for renal tumors [14]. Since then, many reports have been published on RFA for renal tumors and have shown favorable outcomes in terms of local tumor control as well as preservation of renal function [15-18].
We have been performing both percutaneous and laparoscopic RFA on select patients since August 2004 and have been following these patients through serial laboratory assessments, imaging studies, and repeated biopsies of ablated lesions. Herein, we report our experience with RFA of SRMs in the management of 49 patients over the past 31.7 months.
MATERIALS AND METHODS
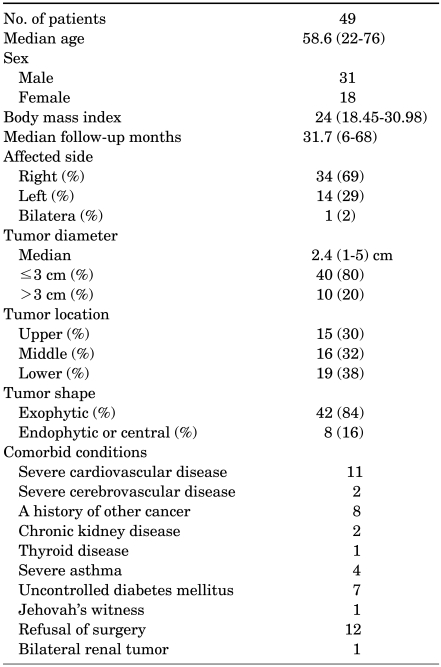
Since August 2004, 49 patients with renal tumors were treated with either percutaneous or laparoscopic RFA. All patients underwent preoperative imaging with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and were diagnosed with RCC. Forty patients underwent percutaneous RFA and nine patients underwent laparoscopic-assisted RFA. Various reasons for undergoing RFA included coexistent morbidities, bilateral renal tumors, high surgical or anesthetic risks, religious observances, and patient preference. Patient demographics and tumor characteristics are shown in Table 1. Patients were informed about other treatment options as well as perioperative complications and morbidities associated with RFA. The percutaneous approach was performed under both sonographic and CT guidance in 40 patients, and laparoscopic-assisted ultrasound-guided RFA was performed in 9.
TABLE 1.
Patient demographics and tumor characteristics of 49 patients
Percutaneous RFA was performed in a prone or modified lateral position according to tumor location. The patients were sedated intravenously with 24 mg of midazolam hydrochloride (Roche, Fontenaysous-Bois, France) and 100 to 300 mg of fentanyl citrate (Hana Pharm. Co., Hwasung, Korea). Local analgesia with 2% lidocaine (Huons, Hwaseong, Korea) was administered in combination with intravenous analgesia. For the laparoscopic-assisted approach, general anesthesia using 100 to 300 mg of propofol (Dongkook Pharm Co., Jincheon, Korea) was conducted in 9 patients. A three- to four-port transperitoneal approach was used to perform laparoscopic-assisted RFA. The kidney-surrounding tumor was exposed and the perirenal fat covering the tumor was removed and sent for pathology. A steerable laparoscopic ultrasound probe was introduced to visualize the tumor size and location. The electrode probe was placed in the deepest part of the renal tumor under real-time laparoscopic ultrasound guidance. RFA was performed with a 200 W generator (Radionics, Burlington, MA, USA) and a single (with one 2.0-3.0 cm tip) internally cooled electrode (Radionics, Burlington, MA, USA) with impedance-controlled pulsed current. The selection of the tip size was based on tumor size and location. Ablation time was a maximum of 12 min for one cycle, and the ablation cycle was repeated if the target temperature achieved was suboptimal. The guidance method used was under sonography and CT for the percutaneous approach. On the basis of the size and location of the tumor, overlapping ablations were performed in some patients by repositioning the electrode to completely ablate the entire tumor. The follow-up for each patient included a physical examination, chest radiography, liver function tests, and a contrast-enhanced CT or MRI. When CT was used for guidance, a contrast-enhanced CT was performed to confirm complete ablation of the lesion and the procedure was terminated. For evaluation of therapeutic efficacy, the absence of enhancement inside the tumor was taken to indicate technical success. If the tumor was enhanced, RFA was repeated. The ablation zone was defined by measuring the difference in the minimal contrast enhancement (i.e., <20 Hounsfield units for CT) as observed on a contrast-enhanced CT after the ablation. Benign periablation enhancement was defined as a thin, concentric, and uniform rim peripheral enhancement with smooth inner margins on a contrast-enhanced CT. On the other hand, an irregular peripheral enhancement was defined as a scattered, nodular, or eccentric peripheral enhancement on a follow-up CT. A contrast-enhanced follow-up CT was performed to assess technical success and effectiveness, ablation zone, benign periablation enhancement, irregular peripheral enhancement, and complications. A follow-up CT was conducted at intervals of 1 day, 1 week, 1 month, 3 and 6 months, and then every 6 months thereafter. A follow-up CT at 1 day and 1 week was conducted to assess technical success and perioperative complications. One-month follow-up CT was performed to determine whether there was any remnant or residual enhancement of the ablated lesion. The technical success rate was defined as complete ablation of the tumor following the initial procedure or additional sessions within a 1-month follow-up. Recurrence was defined as growth of the tumor or any new enhancing portions at 3 months after confirmed nonenhancement of the initial RFA lesion. Complications were categorized into major and minor complications.
RESULTS
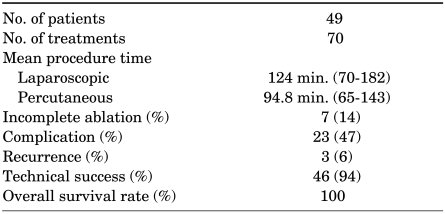
The RFA results are summarized in Table 2. RFA was performed a total of 70 times in 49 patients and technical success was achieved in 46/49 cases (94%) (Fig. 1). The mean tumor size was 2.4 cm (range, 1-5.0 cm), and 42 cases (84%) were exophytic. The mean follow-up period was 31.7 months (range, 6-68 months). The mean procedure time was 94.8 minutes (range, 65-143 minutes) for the percutaneous approach and 124 minutes (range, 70-182 minutes) for the laparoscopic-assisted approach. In a follow-up CT, all tumors revealed a variable degree of size reduction compared to the pretreatment CT.
TABLE 2.
Results of RFA procedures in 49 patients
RFA: radiofrequency ablation
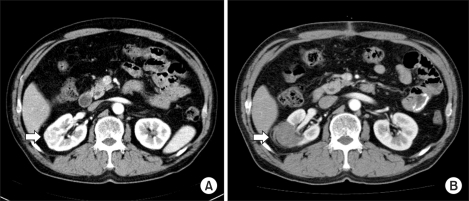
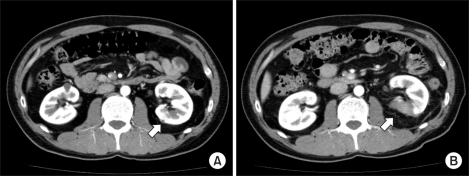
FIG. 1.
Well ablated, small intraparenchymal renal cell carcinoma in a 62-year-old male who underwent RFA. (A) Contrast-enhanced CT scan before RFA demonstrates a 3.3 cm solid enhancing intraparenchymal renal tumor located at the midpolar region of the right kidney. (B) Three-month follow-up contrast-enhanced CT scan demonstrates no periablation enhancement or residual contrast enhancement within the tumor bed, indicating technical success.
Of 49 patients, repeated RFA was necessary in 7 patients (14%) owing to irregular peripheral enhancement, indicating incomplete ablation on follow-up contrast-enhanced CT. As a result, an additional RFA procedure was mandated in one patient on day 1, two patients at 1 week, one patient at 2 weeks, one patient at 1 month, 1 patient at 2 months, and 1 patient at 3 months on the basis of contrast-enhanced CT. A benign periablation enhancement was observed in five patients but subsequently resolved within 3 months of ablation.
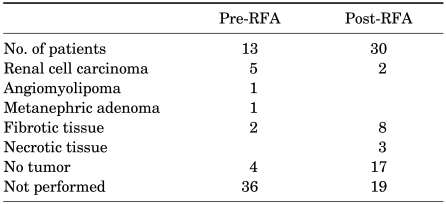
A pre-RFA biopsy was taken in 13 patients who showed conspicuous findings of other possible renal tumors on the preoperative kidney CT. Five patients were diagnosed with RCCs, one with angiomyolipoma, and one with metanephric adenoma, and six patients had fibrotic tissue or normal renal parenchyme. A pre-RFA biopsy was not performed in the remaining 36 patients because of clear radiographic findings of RCC or angiomyolipoma, the possible risk of bleeding, the small size of the lesion, or patient reluctance. To confirm the pathologic criteria of complete ablation, 30 patients underwent 6-month or 1-year follow-up biopsy (Table 3). Follow-up biopsy was not performed in the remaining 19 patients because of a previous renal biopsy result of benign tumors, the patients refused, and the possible risk of bleeding.
TABLE 3.
Results of tumor biopsy
RFA: radiofrequency ablation
Three patients were found to have recurrence at various follow-up intervals. Two patients were discovered to have recurrent tumors on follow-up biopsy. One patient had a viable renal tumor at the 6-month follow-up biopsy despite non-enhancement of the ablated lesion at the 6-month follow-up CT. This patient underwent repeated RFA successfully and no signs of local recurrence were observed at the 27-month follow-up. Another patient who had been treated with RFA for bilateral renal tumors was discovered to have a hyperechoic lesion of the left upper pole of the kidney at the 20-month follow-up ultrasound. Subsequent renal biopsy confirmed a recurrent tumor and the patient underwent RN. Another patient who had undergone successful RFA for a left low renal tumor developed a new cystic renal tumor with enhancement on the midpole of the left kidney at the 30-month follow-up CT. Repeated RFA was performed successfully and no recurrence has been observed for 11 months since the last RFA (Fig. 2). No distant metastasis developed during the mean follow-up of 31.7 months.
FIG. 2.
A recurrent cystic RCC from the left kidney middle pole in a 63-year-old male. (A) Thirty-month follow-up contrast-enhanced CT scan shows suspicious renal cyst with enhancement, left kidney midpole. (B) One day after 2nd RFA follow-up contrast-enhanced CT scan demonstrates no periablation enhancement or residual contrast enhancement within the tumor bed.
Concerning the effect of RFA on preserving renal function, serum creatinine levels did not change compared with those before RFA. Four patients showed a slight increase in creatinine levels (from 1.2 to 1.5 mg/dl, from 1.3 to 1.7 mg/dl, from 1.1 to 1.5 mg/dl, and from 1.3 to 1.6 mg/dl); however, levels returned to baseline levels after 7 to 10 days.
Twenty-three patients (47%) experienced complications (Table 4). The most common complication was perinephric hematomas, which occurred in 13 patients and were managed conservatively. Gross hematuria was seen in 3 patients but all resolved within a few days. Perinephric fluid collection was seen in 3. One patient had a mild thermal injury of the psoas muscle. Another patient suffered from bowel injury with mild hydronephrosis during laparoscopic-assisted RFA. This patient underwent ileostomy diversion and, later, the ileostomy was reverted after 4 months and mild hydronephrosis was resolved within 1 month. Another patient had thermal injury to the liver that was managed with conservative treatment. In addition, another patient had a small amount of contrast leakage into the perirenal space suggesting thermal injury of the pelvocaliceal system at the 1-year follow-up CT. This patient was managed conservatively without any adverse effects. Serum creatinine levels were below the normal range in this patient and no symptoms were observed in the subsequent follow-up.
TABLE 4.
Complications occurring during and after RFA in 49 patients
RFA: radiofrequency ablation
DISCUSSION
Recent advancements in radiologic imaging have led to the increased incidence of small and localized renal tumors. Refinements in surgical techniques with better imaging modalities have resulted in an evolution of nephron-sparing surgeries such as open and laparoscopic partial nephrectomy [9-12].
RFA was initially introduced to treat selected patients who had high surgical or anesthetic risk, a solitary tumor, or multifocal renal tumors. Various reports on local tumor control have so far been promising [15-18]. In the present series, we achieved complete control of renal tumors in 94% of cases with a mean follow-up of 31.7 months, which is quite comparable other series.
The outcomes of RFA are affected by the following factors: tumor size and location, tissue impedance, ablation time, amount of energy delivered, and surface area of the electrodes. Gervais et al reported in a multivariate analysis of 85 patients who underwent percutaneous RFA that small tumor size and a noncentral location of the tumor were independent significant predictors of complete necrosis after a single RFA session [15]. Furthermore, Mylona et al reported a complete response of 85.7% for tumors less than 3 cm after first RFA but reported a noticeably smaller response rate with tumors greater than 5 cm in size [19]. In our experience, tumors greater than 3 cm were technically challenging to completely ablate with only one ablation and required multiple overlapping ablations. Therefore, smaller renal tumors are ideal candidates for obtaining a complete response at first RFA.
Tumor location may also influence ablative outcome. It is reported that the ablative effect on a centrally located tumor is lower because of the heat sink effect of central blood vessels near the renal hilum, in which a regional vascular flow reduces the extent of the thermally induced coagulation [15,20]. By contrast, the ablative effect on exophytic tumors is higher because they are easy to target with the RFA probe and because of the insulating effect of the surrounding perirenal fat, which allows the achievement of higher temperatures during RFA [15,20].
In our study, local tumor control was similarly worse for tumors adjacent to the renal hilum. Also, local tumor control for tumors located in the upper pole of the kidney tended to be less favorable than that for tumors located in the middle or lower pole. This outcome may be due to the difficulty of needle insertion at the upper pole of the kidney, which is always covered by the ribs, spleen, lung, and liver. However, whether the tumor was exophytic or endophytic, this was not related to the outcome except when the tumor was endophytic in a medial central location.
We performed RFA using percutaneous CT guidance. Percutaneous CT guidance allows more reliable electrode placement, and the gas production at the ablation site does not obscure target lesion for additional overlapping ablation.
For patients with difficult access, such as patients with multiple lesions in a solitary kidney, an intervening lung parenchyma or bowel, or thin patients with an anterior lesion, laparoscopy-assisted ultrasound guidance or open intraoperative RFA should be considered as a treatment option. In our study, nine patients were treated with a laparoscopy-assisted ultrasound-guided RFA to isolate the targeted tumors away from adjacent normal structures, such as liver, colon, spleen, or psoas muscle.
The criteria of therapeutic response were based on the report by Goldberg et al [21]. Complete response was defined as the absence of any enhancement within the tumor as observed in the contrast-enhanced CT or MRI. A benign periablation enhancement, which can measure up to 12 mm, typically suggests a transient benign physiologic response to a thermal injury and may persist up to 3 months after the ablation. An irregular peripheral enhancement represents a residual tumor that may occur at the ablative margin. This lesion grows in a scattered, nodular, or eccentric pattern, which indicates incomplete local control. In our study, irregular peripheral enhancement was noted in 12 tumors at 1 day and 1 week. Five patients were found to have benign artifact on follow-up. The other seven patients underwent additional RFA sessions over period of 1 to 3 months.
Recently, the importance of post-RFA biopsy was demonstrated by Weight et al [22]. In 6 of 24 patients who underwent post-RFA biopsy, viable tumor cells were revealed in patients who otherwise had no evidence of enhancement on follow-up CT or MRI. The same authors saw no viable tumors at their 6-month follow-up biopsy after cryoablation and no contrast enhancement on follow-up CT. Thus, the authors concluded that postcryoablation contrast enhancement was a reliable tool and that follow-up biopsies were of low value, whereas after RFA, radiologic findings were not reliable and follow-up biopsies had an impact on further decision making. The authors proposed biopsy of ablated sites in RFA follow-up protocols. Also, Arima et al examined ablated tumor specimens removed 6 weeks after RFA and discovered that the tumor specimens showed well-preserved cancer cells under hematoxylin and eosin staining. However, almost all the cells were stained with terminal dUTP nick end labeling (TUNEL) staining, which is used to detect apoptosis [23]. We have been performing follow-up biopsy after initial RFA. Thirty patients underwent 6-month or 1-year follow-up biopsy. Two patients were discovered to have recurrent tumors on follow-up biopsy. The first patient who showed viable tumor cells in the biopsy otherwise demonstrated no evidence of enhancement at the 6-month follow-up CT. This patient underwent repeated RFA and had no signs of recurrence at the 27-month follow-up CT after second the RFA. The second patient had a bilateral renal tumor that was treated with simultaneous bilateral RFA. At the 2-year follow-up CT, the patient showed enhancement of the previously ablated left kidney lesion and underwent laparoscopic RN because the lesion location was very difficult to target. The patient is free of recurrence as demonstrated at the 46-month follow-up CT after RN.
Complications of RFA can be divided into major (requiring intervention) and minor complications (conservative observation). The reported complications include perinephric hematoma, gross hematuria, pyonephrosis, ureteral stricture, damage to adjacent organs, pain, and paresthesias [24,25]. In the present study, 23 (47%) complications occurred, which included perinephric hematoma in 13, gross hematuria in 3, perinephric fluid collection in 3, a mild thermal injury of the psoas muscle, a bowel injury with mild hydronephrosis, a liver injury, and thermal injury of the pelvocaliceal system. Most of the complications were managed conservatively except one. Inadvertent bowel injury with mild hydronephrosis occurred during laparoscopic-assisted RFA and the patient underwent emergent ileostomy after completion of RFA. Serum creatinine levels were measured in all of our patients, but we observed no deterioration of renal function 3 months after RFA compared with preoperative creatinine levels. However, four patients had transient elevation of serum creatinine levels after RFA; these patients had mildly elevated serum creatinine levels preoperatively, and 1 week later, they returned to preoperative levels. The results were similar when the analysis was limited to those patients with preoperative renal impairment.
The local recurrence rate varies from 0% to 11.1% in cases in which technical success is achieved during the initial RFA [15,18,26]. In our patients, there were 3 patients with local recurrence among 49 patients (6%). With a mean follow-up of 31.7 months, no distant metastasis has been observed. In this study, the overall survival rate was 100%. Despite our favorable intermediate follow-up data, a longer follow-up period may be needed to detect recurrence radiologically after RFA. Also, T1a renal masses are generally known to have low malignant potential, and therefore it may take quite a while to have metastatic extension from a local recurrence after RFA.
CONCLUSIONS
Our intermediate data suggest excellent therapeutic outcomes with RFA with achievement of effective local tumor control and preservation of renal function. Although the mean follow-up period in this study was relatively short, our experience with RFA demonstrates that it is a safe and effective treatment for small, localized renal tumors in a selected group of patients.
Footnotes
The authors have nothing to disclose.
References
- 1.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57–60. [PubMed] [Google Scholar]
- 3.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 4.Bosniak MA, Birnbaum BA, Krinsky GA, Waisman J. Small renal parenchymal neoplasms: further observations on growth. Radiology. 1995;197:589–597. doi: 10.1148/radiology.197.3.7480724. [DOI] [PubMed] [Google Scholar]
- 5.Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol. 2008;179:75–79. doi: 10.1016/j.juro.2007.08.156. [DOI] [PubMed] [Google Scholar]
- 6.Asano T, Mizuguchi Y, Horiguchi A, Ito K, Sumitomo M, Kimura F, et al. Retroperitoneoscopic partial nephrectomy using radiofrequency coagulation for small renal tumors. Urology. 2007;70:869–872. doi: 10.1016/j.urology.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Pyo P, Chen A, Grasso M. Retroperitoneal laparoscopic partial nephrectomy:surgical experience and outcomes. J Urol. 2008;180:1279–1283. doi: 10.1016/j.juro.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herr HW. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup. J Urol. 1999;161:33–34. doi: 10.1016/s0022-5347(01)62052-4. [DOI] [PubMed] [Google Scholar]
- 10.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–445. [PubMed] [Google Scholar]
- 11.Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Park H, Byun SS, Kim HH, Lee SB, Kwon TG, Jeon SH, et al. Comparison of laparoscopic and open partial nephrectomies in T1a renal cell carcinoma: A Korean Multicenter Experience. Korean J Urol. 2010;51:467–471. doi: 10.4111/kju.2010.51.7.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasticier G, Timsit MO, Badet L, De La Torre Abril L, Halila M, Fassi Fehri H, et al. Nephron-sparing surgery for renal cell carcinoma: detailed analysis of complications over a 15-year period. Eur Urol. 2006;49:485–490. doi: 10.1016/j.eururo.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Zlotta AR, Wildschutz T, Raviv G, Peny MO, van Gansbeke D, Noel JC, et al. Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol. 1997;11:251–258. doi: 10.1089/end.1997.11.251. [DOI] [PubMed] [Google Scholar]
- 15.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto ED, Johnson DB, Ogan K, Trimmer C, Sagalowsky A, Margulis V, et al. Short-term efficacy of temperature-based radiofrequency ablation of small renal tumors. Urology. 2005;65:877–881. doi: 10.1016/j.urology.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Farrell MA, Charboneau WJ, DiMarco DS, Chow GK, Zincke H, Callstrom MR, et al. Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol. 2003;180:1509–1513. doi: 10.2214/ajr.180.6.1801509. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Anderson JK, Matsumoto ED, Lotan Y, Josephs S, Cadeddu JA. Radiofrequency ablation of renal tumors: intermediate-term results. J Endourol. 2006;20:569–573. doi: 10.1089/end.2006.20.569. [DOI] [PubMed] [Google Scholar]
- 19.Mylona S, Kokkinaki A, Pomoni M, Galani P, Ntai S, Thanos L. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with solitary kidney: 6 years experience. Eur J Radiol. 2009;69:351–356. doi: 10.1016/j.ejrad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Watkins TW, Parkinson R. Percutaneous radiofrequency ablation of renal tumors: case series of 11 tumours and review of published work. Australas Radiol. 2007;51:412–419. doi: 10.1111/j.1440-1673.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323–331. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 22.Weight CJ, Kaouk JH, Hegarty NJ, Remer EM, O'Malley CM, Lane BR, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008;179:1277–1281. doi: 10.1016/j.juro.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 23.Arima K, Yamakado K, Kinbara H, Nakatsuka A, Takeda K, Sugimura Y. Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: results at 2-year mean follow up. Int J Urol. 2007;14:585–590. doi: 10.1111/j.1442-2042.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 24.Aron M, Gill IS. Renal tumor ablation. Curr Opin Urol. 2005;15:298–305. doi: 10.1097/01.mou.0000177684.93531.85. [DOI] [PubMed] [Google Scholar]
- 25.Kwan KG, Matsumoto ED. Radiofrequency ablation and cryoablation of renal tumours. Curr Oncol. 2007;14:34–38. doi: 10.3747/co.2007.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol. 2007;189:429–436. doi: 10.2214/AJR.07.2258. [DOI] [PubMed] [Google Scholar]