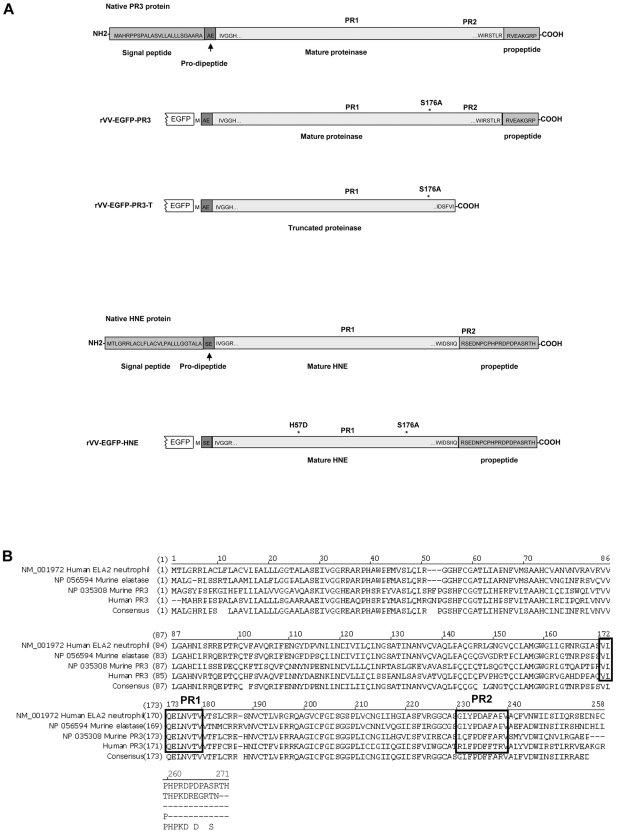
Figure 1.
Structures and sequences of PR3 and HNE proteins. (A) Native forms of PR3 and HNE are shown as well as versions engineered for expression as fusions to the C-terminal of EGFP in rVV. Amino acid sequences are shown at the termini of the mature proteases as well as within the signal peptide, pro-dipeptide, and propeptide segments cleaved off during cellular processing (reviewed in Korkmaz et al1). The positions of the PR1 and PR2 epitopes are indicated in addition to amino acid substitutions introduced to inactivate the enzymatic activity. (B) Alignment of amino acid sequences and consensus of human and murine proteinase 3 and neutrophil elastase ORFs. The alignment uses the unprocessed forms of the polypeptides with N-terminal signal peptides, prodipeptides, and C-terminal propeptides still present. The regions of the PR1 epitope (completely conserved across the 4 proteins) and the PR2 epitopes (nonconserved) are indicated in black boxes.