Figure 4. Inhibition of SHIV replication.
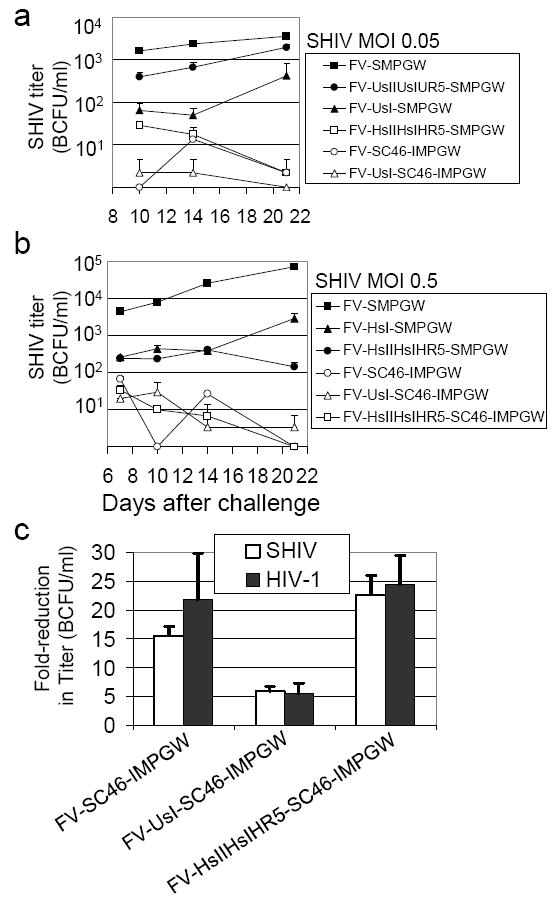
(a) Human CEM.NKR-R5 lymphocytes were transduced with foamy vectors, selected with O6BG and BCNU then sorted to over 95% EGFP-expressing cells and challenged in triplicate with SHIV KU-1 at an MOI of 0.05. At the indicated times after challenge the titer of SHIV in the supernatant was determined by MAGI-CCR-5 assay (BCFU is blue cell focus-forming unit). Zero titers are plotted on the X axis. (b) Human CEM.NKR-CCR5 lymphocytes cells were transduced with the indicated vectors but the challenge virus MOI was increased to 0.5 (c) The three best anti-HIV vectors were evaluated for SHIV and HIV-1 inhibition using a single-cycle replication assay where MAGI-CCR-5 cells were transduced with the indicated vectors, sorted to over 98% EGFP-positive, then challenged and tested for virus replication 48 hours after challenge. The fold-reduction in detected infectious units is reported for each vector relative to the control vector FV-SMPGW that does not contain an anti-HIV transgene.
