Abstract
The function of an implanted tissue-engineered pancreatic construct is influenced by many in vivo factors; however, assessing its function is based primarily on end physiologic effects. As oxygen significantly affects cell function, we established a dual perfluorocarbon method that utilizes 19F nuclear magnetic resonance spectroscopy, with perfluorocarbons as oxygen concentration markers, to noninvasively monitor dissolved oxygen concentration (DO) in βTC-tet cell-containing alginate beads and at the implantation milieu. Beads were implanted in the peritoneal cavity of normal and streptozotocin-induced diabetic mice. Using this method, the feasibility of acquiring real-time in vivo DO measurements was demonstrated. Results showed that the mouse peritoneal environment is hypoxic and the DO is further reduced when βTC-tet cell constructs were implanted. The DO within cell-containing beads decreased considerably over time and could be correlated with the relative changes in the number of viable encapsulated cells. The reduction of construct DO due to the metabolic activity of the βTC-tet cells was also compatible with the implant therapeutic function, as observed in the reversal of hyperglycemia in diabetic mice. The importance of these findings in assessing implant functionality and host animal physiology is discussed.
Introduction
Insulin-dependent diabetes mellitus is an autoimmune disease resulting from the destruction of insulin-producing β-cells of the pancreatic islets. Cell-based therapies are being explored as improved treatments relative to insulin injections, as they have the potential to provide tight regulation of blood glucose levels through physiologic secretion of insulin. Considerable research is being done in developing tissue-engineered pancreatic constructs where islets or other insulin secreting cells are encapsulated in biocompatible materials for immune protection and improved in vivo survival postimplantation.1–4
Despite advances, the challenge of engineering a long-term functional pancreatic construct remains, as the causes and mechanisms of implant failure are numerous and poorly understood.5–8 Immune rejection is a main obstacle in successfully transplanting allogeneic or xenogeneic cells even with the current encapsulation systems available.7,9,10 Several investigators have reported on the infiltration of large numbers of host immune cells in the peritoneal cavities of rats and mice during the rejection of encapsulated allografts and xenografts.9,11–13 Rejection due to an immune response can be related to direct cytokine-mediated damage, not prevented by the encapsulation material; it could also be related to a significant inflammatory or fibrotic reaction toward the implant, which hampers the transport of nutrients, including oxygen, toward the implanted cells.7,9,14 The problem of limited construct oxygenation may be exacerbated by the low oxygen at the implantation site, and, in many cases, the absence of vascularization around these implants.7,15,16
Currently, assessment of the in vivo function of a pancreatic construct relies on measurements of blood glucose and insulin or C-peptide levels during oral glucose challenges.17 A direct evaluation of the physiological state of a construct can only be done at the experimental end-point; therefore, to obtain information on the dynamic changes occurring in vivo, animals need to be euthanized and constructs retrieved at various time points. Besides the large number of animals needed for such studies, the large inter-animal variability presents additional challenges. Thus, there are increasing efforts toward developing methods for evaluating in vivo construct function on the same animal in real-time, in a minimally or noninvasive way.17–20 As construct oxygenation significantly affects cell survival and function,7,21–23 monitoring of oxygen levels in pancreatic implants could provide direct information on their functionality. However, most reports on the effect of hypoxia on construct function are limited to in vitro settings22,24–27; in vivo evaluation of hypoxia is currently performed through indirect methods postexplantation.15,16,28
We are therefore pursuing the development of a noninvasive method of monitoring dissolved oxygen concentration (DO) in a tissue-engineered pancreatic construct using 19F nuclear magnetic resonance (NMR) spectroscopy, with perfluorocarbons (PFCs) as oxygen concentration markers. The principle behind this method is the linear relationship between the inverse spin lattice relaxation time of the fluorine resonances from PFCs and oxygen tension.29 We recently developed a dual PFC method consisting of incorporating a different PFC in cell-containing experimental beads and cell-free control beads; the latter group of beads was used to monitor the DO at the implantation milieu, as instantaneous equilibration of intrabead and external DO could be assumed. Measuring DO within the experimental implant offers an assessment of the metabolic activity of the cells within the construct.26 In a previous study, we demonstrated the capability of this method to acquire real-time DO measurements within alginate beads implanted in the peritoneal cavity of normal mice and to acquire correlations between changes in intrabead DO and growth of the encapsulated cells or attachment of host cells to the beads, the latter two evaluated postexplantation.30
In this study, we expand on our previous work by implementing the dual PFC method to measure DO levels in βTC-tet cell-containing barium alginate beads implanted in the peritoneal cavity of diabetic mice. Our goals were to obtain real-time measurements of construct DO in a diabetic mouse model, and to correlate these measurements to the therapeutic function of the implant. Additionally, we investigated the effect of the implanted cell constructs on the peritoneal DO and compared the peritoneal DO of normal and diabetic mice. The usefulness of the information provided by these studies on the in vivo functionality of encapsulated cell implants, and on the effect of such implants on host animal physiology, is discussed.
Materials and Methods
Cell culture, PFC preparation, and cell/PFC encapsulation
Murine insulinoma βTC-tet cells31 were obtained from the laboratory of Dr. Shimon Efrat, Albert Einstein College of Medicine (Bronx, NY). Cells were cultured as monolayers in T-175 flasks, in a 37°C, 5% CO2 humidified incubator. Culture medium consisted of Dulbecco's modified Eagle's medium (DMEM; Sigma Chemical Co., St. Louis, MO) with 25 mM glucose, supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% L-glutamine to a final concentration of 6 mM, and it was changed every 2–3 days. Cell monolayers that reached 80% to 90% confluency were split by treatment with 0.25% trypsin-EDTA (Sigma Chemical Co.); passage number increased by one at each splitting. Cells of passage number 37–42 were used in this study.
The PFCs used were perfluorotributylamine (PFTBA) and perfluoro-15-crown-5-ether (PFCE). The PFC emulsion was prepared following a protocol adapted from Joseph et al.32 and described by Gross et al.26 The emulsion was added to 3% w/v low viscosity, high mannuronic content alginate (LVM; NovaMatrix, Drammen, Norway) at a concentration of 50 μg/mL, and mixed thoroughly. Cells were encapsulated in barium alginate beads as described previously.30 Briefly, cells of 90% confluency were harvested by trypsin-EDTA (MediaTech, Manassas, VA). Viable cell counts were performed using trypan blue (Sigma Chemical Co.) exclusion method and cells were washed twice with unsupplemented DMEM. The cell pellet was resuspended at a density of 2×107 cells/mL in the alginate/PFC solution. The alginate/PFC/cell suspension was then extruded via a 0.5 mm needle into a 30 mM BaCl2 solution using an electrostatic bead generator (Nisco Engineering, Inc., Zurich, Switzerland) to form barium alginate beads. Beads were allowed to crosslink in the BaCl2 solution for 5 min and were then washed 5 times with physiological saline and three times with unsupplemented DMEM. The average diameter of the resulting beads after washing was 700±100 μm.
For one of the control groups (control 2, see below), encapsulated cells were killed through heating at 60°C for half an hour. Cell death was confirmed through trypan blue (Sigma Chemical Co.) exclusion method before implantation. The mechanical integrity of the beads was determined by measuring the Young's modulus via uniaxial compression using a Bose ElectroForce® 3100 apparatus (EnduraTec, Inc., Eden Prairie, MN) and was found not to be compromised by the heating process.33
Animals
All experiments were carried out in accordance with protocols approved by the Georgia Tech Animal Care and Use Committee. Seven-week-old Balb/c mice were obtained from Jackson Labs (Bar Harbor, ME) and housed under specific pathogen-free conditions in the animal facility at Georgia Tech. Mice that reached 10 weeks of age were made diabetic through multiple (two to three dosages every 3 days) i.p injections of 210 mg/kg of streptozotocin (Sigma Chemical Co.), dissolved in sodium citrate buffer (pH 4.5) (Sigma Chemical Co.). This animal model of chemically induced diabetes does not have autoimmune features; however, it is a widely used diabetes model, which was quite appropriate for the purposes of this study. Mouse blood glucose levels and body weights were monitored daily. Glucose concentrations were measured in blood samples collected from tail veins using TRUEtrack glucose monitors (CVS, Woonsocket, RI). Mice were considered diabetic when their blood glucose levels were above 350 mg/dL for 2 consecutive days.
Construct implantation
Barium alginate beads were implanted in the mouse peritoneal cavity through a simple injection. A total of 17 diabetic mice (experimental) received each 0.6 mL of cell-free PFTBA beads and 0.2 mL of cell-containing PFCE beads (corresponding to 4×106 implanted βTC-tet cells); a total of four normal mice (control 1N) and six diabetic mice (control 1D) received each 0.7 mL of cell-free PFTBA beads and 0.3 mL of cell-free PFCE beads; and a total of three normal mice (control 2N) and four diabetic mice (control 2D) received each 0.6 mL of cell-free PFTBA beads and 0.2 mL of dead cell-containing PFCE beads (corresponding to 4×106 implanted dead βTC-tet cells). As βTC-tet cells undergo growth arrest in the presence of tetracycline,31 all mice were given drinking water containing 4 mg/mL tetracycline (Sigma Chemical Co.) with 1.5% (w/v) sucrose (Sigma Chemical Co.) starting sometime between days 1 and 4 until the end of the experiment to prevent excessive cell proliferation.
Construct DO measurements
19F NMR spectra were acquired using a 16-mm-diameter surface coil (Doty Scientific, Columbia, SC) on a 7 T horizontal bore magnet equipped with a Bruker Avance console (Bruker, Billerica, MA) at the Nanomedicine Research Institute Magnetic Resonance Laboratory, Georgia Tech. An inversion recovery sequence with a 180° adiabatic pulse (hyperbolic secant) was used to acquire T1 relaxation measurements; there were 23 delay times ranging from 0.01 s to 7 s for each T1 determination. Acquisition parameters for the experiments were 2 transients, 4096 data points per free induction decay, 15 s relaxation delay. Peak intensity was extracted using the area under the peak, and all T1 relaxation rates were determined through a three-parameter exponential fit using Bruker Avance software.
Calibration of the inverse T1 versus DO was performed by loading cell-free alginate beads containing 50 mg/mL of PFTBA or PFCE emulsion in a 50 mL centrifuge tube containing physiologic saline. The solution was bubbled with nitrogen, air, or oxygen at 37°C and atmospheric pressure for at least 45 min to allow for equilibration, and then sealed and placed on the surface coil. Temperature was maintained by circulating water from a bath through tubing wrapped around the centrifuge tube. T1 relaxation measurements were correlated to DO as follows:
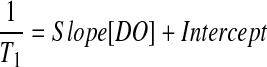 |
During the NMR scans of mice, the animals were anesthetized in 1.5% isoflurane in oxygen flowing at 1.5 L/min, and kept at 37°C with a warming pad.
Postexplantation studies
After NMR scans, experimental mice were euthanized on days 4, 8, and 16, and control diabetic and normal mice were euthanized on days 2 and 16, respectively. Beads were explanted via peritoneal lavage with Dulbecco's phosphate-buffered saline (MediaTech). Explanted bead integrity was evaluated through light microscopy. The relative changes in the number of viable cells within the beads were estimated based on a qualitative assessment of cell viability and the presence of cell clusters, through live/dead staining and histology or confocal microscopy. Host cell attachment to the constructs was also assessed through these assays. For viability analysis, living and dead cells were stained with calcein-AM (excitation: 488 nm/emission: 500–530 nm) and ethidium homodimer (excitation: 543 nm/emission: 560 nm) (Invitrogen, Eugene, OR) and observed using a confocal microscope (LSM 510 META; Zeiss, Maple Grove, MI). For histological analysis, beads were fixed in 2% glutaraldehyde (Sigma Chemical Co.), embedded in paraffin, sectioned, and stained with hematoxylin/eosin.
To evaluate β-cell function, retrieved beads were subjected to an insulin secretion test, where the beads were cultured in basal medium (0 mM glucose, unsupplemented DMEM) for 1 h and then transferred to stimulating medium (16 mM, fully supplemented DMEM) for 30 min. Insulin concentration was assayed using mouse insulin ELISA (Mercodia, Uppsala, Sweden) with absorbance measured at 450 nm using a Spectra Max Plus Plate Reader (Molecular Devices, Sunnyvale, CA). As the explanted bead samples were random mixtures of cell-free and cell-containing beads and contained varying numbers of host cells, the number of βTC-tet cells could not be quantified; therefore, only the ranges of the stimulated secretion results are reported.
Statistical analysis
All data were analyzed using Minitab software (Minitab, Inc., State College, PA). A total of 3 T1 relaxation measurements were obtained from a single mouse and the DO was determined as the average of these values. The DO data are reported as the mean of the average DO values from the mice scanned on a particular day±the standard deviation of the averages. The significance of DO differences between groups and days were evaluated using one-way analysis of variance with general linear model.
Results
Correction of diabetes in streptozotozin-induced diabetic Balb/c mice
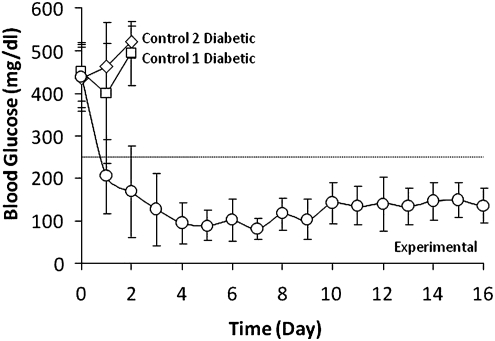
The construct therapeutic function was evaluated through daily determinations of random blood glucose levels in mice. Figure 1 shows blood glucose concentrations of diabetic mice implanted with encapsulated βTC-tet cells (experimental), cell-free beads (control 1D), and encapsulated dead βTC-tet cells (control 2D). Implantation of βTC-tet cells corrected hyperglycemia within 3 days and maintained normoglycemia in the mice until euthanasia, indicating that the constructs were functional within the experimental time frame. As tetracycline regulated βTC-tet cell proliferation, the mice did not become hypoglycemic at later time points but blood glucose levels stabilized within normal ranges. There was a 7% increase in the average body weight of these mice within 16 days. Control diabetic mice that received cell-free beads and dead cell-containing beads remained hyperglycemic and were euthanized 2 days postimplantation.
FIG. 1.
Blood glucose levels of control 1D and 2D mice receiving cell-free beads (n=6) and dead cell-containing beads (n=4), respectively, and of experimental mice receiving βTC-tet cell-containing beads (n=9 to 17). On day 4, five experimental mice were euthanized; on day 8, three mice experimental were euthanized.
Monitoring DO in the tissue construct and the peritoneal cavity
The inverse T1 relaxation measurements of 19F resonances increased linearly with DO. The fitting of the calibration curves yielded a slope and intercept of 2.10 and 0.85 for PFCE (R2=1) and 2.04 and 0.44 for PFTBA (R2=0.999), respectively; these parameters were used for DO determinations from T1 measurements of the implanted constructs.
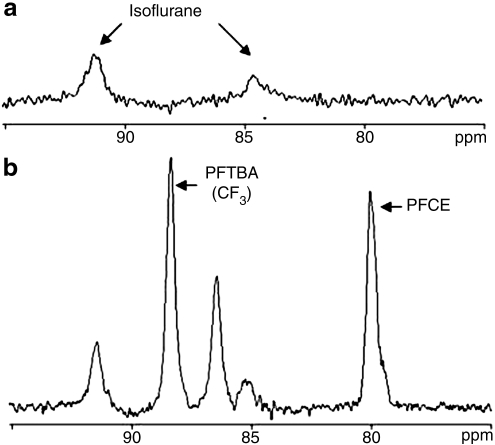
Figure 2 shows typical 19F NMR spectra acquired from an anesthetized mouse implanted with cell- and PFC-free barium alginate beads (Fig. 2a) and a mouse implanted with cell-free, PFTBA- and PFCE-containing barium alginate beads (Fig. 2b). There were two resonances identified to be from isoflurane, which, however, did not interfere with those from PFCs. The PFCE peak was approximately 9 ppm apart from CF3 of PFTBA; the chemical shift proximity of these two peaks allowed for simultaneous T1 acquisitions.
FIG. 2.
19F nuclear magnetic resonance spectrum of an anesthetized mouse implanted with (a) cell- and perfluorocarbon (PFC)-free alginate beads and (b) cell-free, perfluorotributylamine (PFTBA) and perfluoro-15-crown-5-ether (PFCE)-containing alginate beads. PFCE has a single resonance peak and PFTBA exhibited four resonance peaks (only two are displayed in the figure); measurements were based on the CF3 resonance, which is the predominant peak. A higher volume of PFTBA vs. PFCE beads was implanted, as PFTBA-CF3 displays a lower intensity peak when compared to PFCE when the two compounds are at the same concentration.
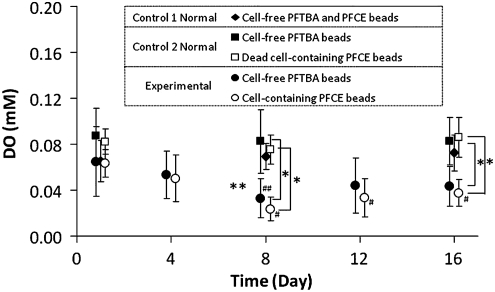
Experimental diabetic mice were implanted in the peritoneal cavity with βTC-tet cell-containing PFCE beads and cell-free PFTBA beads. DO measurements within these two bead populations were obtained over time and compared against those measured in control mice. The purpose of coimplanting cell-free, PFC-containing beads in all groups was to measure the oxygen levels at the implantation milieu. Figure 3 shows the 19F NMR-acquired DO in beads from the experimental and control 1N and 2N mice over a 16 day period.
FIG. 3.
Dissolved oxygen concentration measurements in cell-containing PFCE beads and cell-free PFTBA beads implanted in experimental mice (n=8 on day 1, n=9 on all other days), in cell-free PFCE and PFTBA beads implanted in control 1N mice (n=4), and in dead cell-containing PFCE beads and cell-free PFTBA beads implanted in control 2N mice (n=3). **p<0.05, compared between experimental and control 1N mice, the latter with nested PFC groups; *p<0.05, compared between experimental and control 2N mice; ##p<0.05, compared to day 1 in cell-free beads in experimental mice; #p<0.05, compared to day 1 in cell-containing beads in experimental mice. All comparisons analyzed using one-way analysis of variance with general linear model.
The average DO in the peritoneal cavity of control 1N mice ranged from 0.065 to 0.073 mM during the day 1 to 16 time period, with no statistical differences found between any of the days. The peritoneal DO of control 1D mice (n=6) was also measured on days 1 and 2 and found to be 0.088±0.012 and 0.079±0.016 mM, respectively; the DO on day 1 was not different from that in control 1N mice.
One day postimplantation, the peritoneal DO in experimental mice was 0.065±0.03 mM and in the βTC-tet cell-containing beads 0.063±0.012 mM; these were not statistically different. The DO in the cell-containing beads decreased over time, and on day 8 became and stayed significantly lower than the day 1 value until the end of the experiment. The peritoneal DO was also significantly lower on day 8 than on day 1. The DO in the cell-containing beads was comparable to the peritoneal DO on all days in the experimental mice. Notably, the DO within the cell-containing beads and the peritoneal cavity in experimental mice were both significantly lower than the peritoneal DO in control 1N mice on days 8 and 16.
To assess whether the lowered DO observed in experimental mice was due to the metabolic activity of the implanted βTC-tet cells, or whether recruited host immune cells also played a role, measurements were obtained from control 2N mice implanted with cell-free and dead βTC-tet cell-containing beads. Average DO values in the dead cell-containing PFCE beads ranged from 0.075 to 0.086 from day 1 to 16, with no statistical differences between any of the days. DO measurements were also obtained from the same type of beads implanted in control 2D mice (n=4) on days 1 and 2, and found to be 0.067±0.014 and 0.072±0.016 mM, respectively; the DO on day 1 was not different than that in control 2N mice. DO measurements in the dead cell-containing beads were comparable to the surrounding peritoneal DO on all days for all mice. More importantly, these DO measurements were also comparable with the peritoneal DO in control 1N mice, but significantly higher than the DO measured in beads implanted in experimental mice.
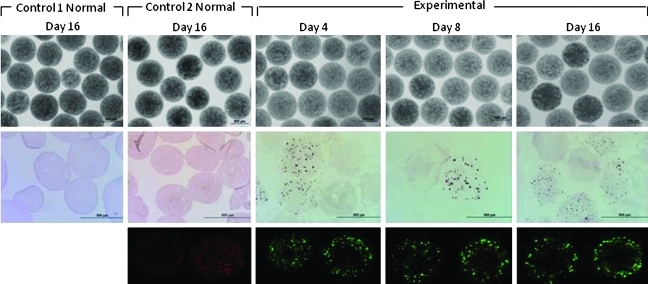
Evaluation of tissue constructs postexplantation
Figure 4 shows representative images of beads explanted from control 1N and 2N mice on day 16 and from experimental mice on days 4, 8, and 16. Explanted beads from all groups were intact when observed microscopically, although a few broken beads were found occasionally. Live/dead staining and histological examination showed that beads from experimental mice contained viable βTC-tet cells on all explantation days, and that both cell-free and cell-containing beads were clear of host cell attachment throughout the experimental time period. Some initial cell growth was observed the first week postimplantation; insignificant cell growth was observed thereafter. Encapsulated βTC-tet cells were also functional on all explantation days, as detected by insulin release in response to an in vitro glucose stimulation test (Table 1). With beads explanted from control mice 2N, no live βTC-tet cells were detected by live/dead staining and histology; also, when beads were subjected to an in vitro glucose stimulation test, no insulin was detected, confirming that the cells were indeed nonfunctional. Similar observations were made with beads explanted from the control mice 1D and 2D on day 2 (images not shown).
FIG. 4.
Light micrographs (4×), histological analysis (10×), and live/dead staining (10×) of representative beads explanted on day 4 (n=5), day 8 (n=3), and day 16 (n=9) from experimental mice and beads explanted on day 16 from control 1N mice (n=3) and control 2N mice (n=4). Color images available online at www.liebertonline.com/tec
Table 1.
Stimulated Insulin Secretion Rates of Encapsulated βTC-tet Cells Explanted from Experimental and Control 2N Mice
| Stimulated insulin secretion rate (pmol/h) | |
|---|---|
| Experimental mice | |
| Day 4 (n=4) | 23.6–93.6 |
| Day 8 (n=3) | 30.8–47.3 |
| Day 16 (n=7) | 28.5–198.5 |
| Control 2N mice | |
| Day 16 (n=4) | Undetected |
Explants consisted of random mixtures of cell-free and cell-containing beads and contained unknown numbers of host cells; hence, data are reported as ranges of the insulin secretion rates measured from all the beads explanted from each mouse.
Discussion
Tissue-engineered pancreatic constructs have the potential to provide tight glycemic control in insulin-dependent diabetes mellitus patients. The peritoneal cavity is a plausible implantation site as it can easily accommodate these constructs.34 However, with beads implanted in the peritoneal cavity, the supply of oxygen to the cells occurs through passive diffusion only, and a host cellular response could further deplete oxygen within the encapsulation device.7 As a multitude of studies have reported on compromised insulin secretory response of islets or transformed β-cell lines under in vitro low oxygen conditions21,22,24,25 and on early postimplantation graft failure as a result of hypoxic stress,7,8,16 the ability to monitor construct oxygenation is evidently important.
While noninvasive evaluation of oxygen supply at different implantation sites has been performed using cell-free PFC constructs,35,36 our work involves the investigation of oxygen levels within functional, cell-containing pancreatic implants. In this study, we demonstrated that in vivo real-time oxygen measurements within a tissue-engineered pancreatic construct, implanted in a diabetic mouse model, can be obtained noninvasively through 19F NMR. Further, the design of the dual PFC method enabled simultaneous tracking of DO within the construct and at the implantation milieu. Measurements confirmed the peritoneal cavity of normal mice to be hypoxic, with DO values comparable to those reported in literature.26,30,35 Equivalent peritoneal DO values were also obtained in diabetic mice within 2 days postimplantation.
In experimental mice implanted with encapsulated βTC-tet cells, DO measurements in the cell-containing beads appeared to correlate with the relative change in the number of viable cells examined postexplantation, as observed in the decrease in DO with cell growth over time. This reduced DO at later time points can be attributed to a higher oxygen consumption by βTC-tet cells, as there was no host cell attachment to any of the beads. Notably, the implantation of viable encapsulated cells resulted in a significant reduction of the peritoneal DO relative to mice that did not receive viable cell implants. Even though there was no inflammatory reaction to the beads, a cellular immune response could still be elicited due to antigens secreted by the βTC-tet cells. Shed antigens can combine with antibodies to form complexes which bind to the receptors on peritoneal macrophages.9,11 If the antigen load is sufficiently high, the recruitment of host peritoneal cells could be significant, possibly contributing to the observed reduction in peritoneal DO.
To assess this possibility, an antigenic load from a similar encapsulation system was introduced by implanting beads containing dead βTC-tet cells in control 2N and 2D mice; dead cells would eventually lyse and hence release antigens. Inevitably, control 2D mice which received such nonfunctional cell implants remained hyperglycemic and were euthanized 2 days postimplantation. In control 2N, the DO within dead cell-containing beads and the peritoneal DO did not decrease over time, but remained comparable to the peritoneal DO of control 1N mice at all measured time points. Therefore, these results indicate that the observed reduction in construct and peritoneal DO in experimental mice was caused by the metabolic needs imposed by the viable implant. Although unlikely, the possibility of a higher recruitment of host cells by the viable βTC-tet cells relative the dead cells cannot be excluded. Nevertheless, regardless of its precise cause, the lowered DO has significant implications with respect to implant survival and function. This is especially critical in situations in which a higher number of implanted cells may be needed, such as with xenografts or with larger animal models.6
Contrary to our in vitro observations,30 no difference was detected between the DO within beads containing metabolically active cells and the surrounding peritoneal DO in experimental mice at all measured time points. This might be due to the lower magnet strength (7 T vs. 11.7 T) and smaller sample volume used for the in vivo studies.
The analysis of construct DO in relation to implant function is important, as the presence of viable islets or transformed β-cells does not ascertain insulin secretory capacity.22,27,37 In this study, the encapsulated βTC-tet cells survived well in the peritoneal cavity and the number of implanted cells was sufficient to restore normoglycemia in diabetic mice. Despite the reduced DO in the cell-containing beads in experimental animals, sufficient insulin secretory capacity was apparently maintained, as normoglycemia was maintained in these mice. This can be attributed to the high tolerance of transformed β-cell lines to hypoxia; for instance, Papas et al. reported insulin secretion of βTC3 being compromised only at an oxygen levels below 0.009 mM.27 The observed implant therapeutic function was compatible with the overall reduction in DO within constructs containing metabolically active βTC-tet cells.
19F NMR is a useful modality that can be utilized to obtain in vivo quantitative DO measurements within implants without relying on indirect measurement methods. Correlations established between construct DO and the observed end physiologic effects may provide a better insight into the in vivo factors that affect implant function and assist in the development of a long-term functional tissue-engineered pancreatic construct for diabetes treatment.
Conclusions
Using 19F NMR, the dual PFC method allowed for real-time, noninvasive in vivo tracking of DO within a tissue-engineered pancreatic construct and at the implantation milieu. Measurements showed that the peritoneal environment is hypoxic in both normal and diabetic mice, and that the peritoneal DO is significantly lower in the presence of metabolically active implanted cells. DO measurements can be used to assess relative changes in the number of viable cells within the construct. The overall reduction in construct DO due to the metabolic activity of βTC-tet cells was compatible with implant function as observed in the reversal of hyperglycemia in diabetic mice.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK76801, DK47858, and DK73991). The authors wish to thank Chun Yong for carrying out the mechanical testing for the beads, Johannes Leisen for his NMR technical assistance, and Dr. Robert Long, Jr., Dr. Nicholas Simpson, and Dr. Susan Safley for many useful discussions.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nerem R.M. Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1:3. doi: 10.1089/ten.1995.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Sambanis A. Tang S.C. Cheng S.Y. Stabler S.L. Long R.C., Jr. Constantinidis I. Core technologies in tissue engineering and their application to the bioartificial pancreas. In: Ikada Y., editor; Umakoshi Y., editor; Hotta T., editor. Tissue Engineering for Therapeutic Use 6. Amsterdam, The Netherlands: Elsevier Science B.V.; 2002. pp. 5–18. [Google Scholar]
- 3.Sambanis A. Bioartificial pancreas. In: Lanza R., editor; Langer R., editor; Vacanti J., editor. Principles of Tissue Engineering. Third. Burlington, MA: Elsevier Academic Press; 2007. pp. 619–633. [Google Scholar]
- 4.Black S.P. Constantinidis I. Cui H. Tucker-Burden C. Weber C.J. Safley S.A. Immune responses to an encapsulated allogeneic islet beta-cell line in diabetic NOD mice. Biochem Biophys Res Commun. 2006;340:236. doi: 10.1016/j.bbrc.2005.11.180. [DOI] [PubMed] [Google Scholar]
- 5.Biarnes M. Montolio M. Nacher V. Raurell M. Soler J. Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 6.Duvivier-Kali V.F. Omer A. Lopez-Avalos M.D. O'Neil J.J. Weir G.C. Survival of microencapsulated adult pig islets in mice in spite of an antibody response. Am J Transplant. 2004;4:1991. doi: 10.1111/j.1600-6143.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 7.de Groot M. Schuurs T.A. van Schilfgaarde R. Causes of limited survival of microencapsulated pancreatic islet grafts. J Surg Res. 2004;121:141. doi: 10.1016/j.jss.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Emamaullee J.A. Shapiro A.M. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 2007;16:1. [PubMed] [Google Scholar]
- 9.Weber C.J. Safley S. Hagler M. Kapp J. Evaluation of graft-host response for various tissue sources and animal models. Ann N Y Acad Sci. 1999;875:233. doi: 10.1111/j.1749-6632.1999.tb08507.x. [DOI] [PubMed] [Google Scholar]
- 10.De Vos P. de Haan B.J. de Haan A. van Zanten J. Faas M.M. Factors influencing functional survival of microencapsulated islet grafts. Cell Transplantation. 2004;13:515. doi: 10.3727/000000004783983738. [DOI] [PubMed] [Google Scholar]
- 11.Cui H. Tucker-Burden C. Cauffiel S.M. Barry A.K. Iwakoshi N.N. Weber C.J. Safley S.A. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation. 2009;88:160. doi: 10.1097/TP.0b013e3181abbfc1. [DOI] [PubMed] [Google Scholar]
- 12.Safley S.A. Kapp L.M. Tucker-Burden C. Hering B. Kapp J.A. Weber C.J. Inhibition of cellular immune responses to encapsulated porcine islet xenografts by simultaneous blockade of two different costimulatory pathways. Transplantation. 2005;79:409. doi: 10.1097/01.tp.0000150021.06027.dc. [DOI] [PubMed] [Google Scholar]
- 13.Safley S.A. Cui H. Cauffiel S. Tucker-Burden C. Weber C.J. Biocompatibility and immune acceptance of adult porcine islets transplanted intraperitoneally in diabetic NOD mice in calcium alginate poly-L-lysine microcapsules versus barium alginate microcapsules without poly-L-lysine. J Diabetes Sci Technol. 2008;2:760. doi: 10.1177/193229680800200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newgard C.B. Hohmeier H.E. Lu D. Jensen M.V. Tran V.V. Chen G. Burgess S. Sherry A.D. Understanding of basic mechanisms of beta-cell function and survival: prelude to new diabetes therapies. Cell Biochem Biophys. 2004;40(3 Suppl):159. doi: 10.1385/cbb:40:3:159. [DOI] [PubMed] [Google Scholar]
- 15.De Vos P. Van Straaten J.F.M. Nieuwenhuizen A.G. de Groot M. Ploeg R.J. De Haan P.J. Van Schilfgaarde R. Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes. 1999;48:1381. doi: 10.2337/diabetes.48.7.1381. [DOI] [PubMed] [Google Scholar]
- 16.Lau J. Henriksnas J. Svensson J. Carlsson P.O. Oxygenation of islets and its role in transplantation. Curr Opin Organ Transplant. 2009;14:688. doi: 10.1097/MOT.0b013e32833239ff. [DOI] [PubMed] [Google Scholar]
- 17.Leoni L. Roman B.B. MR imaging of pancreatic islets: tracking isolation, transplantation and function. Curr Pharm Des. 2010;16:1582. doi: 10.2174/138161210791164171. [DOI] [PubMed] [Google Scholar]
- 18.Constantinidis I. Long R., Jr. Weber C. Safley S. Sambanis A. Non-Invasive monitoring of a bioartificial pancreas in vitro and in vivo. Ann N Y Acad Sci. 2001;944:83. doi: 10.1111/j.1749-6632.2001.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 19.Witkowski P. Sondermeijer H. Hardy M.A. Woodland D.C. Lee K. Bhagat G. Witkowski K. See F. Rana A. Maffei A. Itescu S. Harris P.E. Islet grafting and imaging in a bioengineered intramuscular space. Transplantation. 2009;88:1065. doi: 10.1097/TP.0b013e3181ba2e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stabler C.L. Long R.C., Jr. Constantinidis I. Sambanis A. In vivo noninvasive monitoring of a tissue engineered construct using 1H NMR spectroscopy. Cell Transplant. 2005;14:139. doi: 10.3727/000000005783983197. [DOI] [PubMed] [Google Scholar]
- 21.Papas K.K. Long R.C., Jr. Sambanis A. Constantinidis I. Development of a bioartificial pancreas: II Effects of oxygen on long-term entrapped betaTC3 cell cultures. Biotechnol Bioeng. 1999;66:231. [PubMed] [Google Scholar]
- 22.Dionne K.E. Colton C.K. Yarmush M.L. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 23.Giuliani M. Moritz W. Bodmer E. Dindo D. Kugelmeier P. Lehmann R. Gassmann M. Groscurth P. Weber M. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14:67. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 24.de Groot M. Schuurs T.A. Keizer P.P. Fekken S. Leuvenink H.G. van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12:867. [PubMed] [Google Scholar]
- 25.Papas K.K. Long R.C., Jr. Sambanis A. Constantinidis I. Development of a bioartificial pancreas: I long-term propagation and basal and induced secretion from entrapped betaTC3 cell cultures. Biotechnol Bioeng. 1999;66:219. [PubMed] [Google Scholar]
- 26.Gross J.D. Long R.C., Jr. Constantinidis I. Sambanis A. Monitoring of dissolved oxygen and cellular bioenergetics within a pancreatic substitute. Biotechnol Bioeng. 2007;98:261. doi: 10.1002/bit.21421. [DOI] [PubMed] [Google Scholar]
- 27.Papas K.K. Long R.C., Jr. Constantinidis I. Sambanis A. Effects of short-term hypoxia on a transformed cell-based bioartificial pancreatic construct. Cell Transplant. 2000;9:415. doi: 10.1177/096368970000900312. [DOI] [PubMed] [Google Scholar]
- 28.Miao G. Ostrowski R.P. Mace J. Hough J. Hopper A. Peverini R. Chinnock R. Zhang J. Hathout E. Dynamic production of hypoxia-inducible factor-1alpha in early transplanted islets. Am J Transplant. 2006;6:2636. doi: 10.1111/j.1600-6143.2006.01541.x. [DOI] [PubMed] [Google Scholar]
- 29.Parhami P. Fung B.M. F-19 relaxation study of perfluoro chemicals as oxygen carriers. J Phys Chem. 1983;87:1928. [Google Scholar]
- 30.Goh F. Long R., Jr. Simpson N. Sambanis A. Dual perfluorocarbon method to noninvasively monitor dissolved oxygen concentration in tissue engineered constructs in vitro and in vivo. Biotechnol Prog. 2011;27 doi: 10.1002/btpr.619. [DOI] [PubMed] [Google Scholar]
- 31.Efrat S. Fusco-DeMane D. Lemberg H. al Emran O. Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci U S A. 1995;92:3576. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph P.M. Fishman J.E. Mukherji B. Sloviter H.A. In vivo 19F NMR imaging of the cardiovascular system. J Comput Assist Tomogr. 1985;9:1012. doi: 10.1097/00004728-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee I.N. Department of Chemical and Biomolecular Engineering, Georgia Institute of Technology; Atlanta, GA: 2008. A rational design approach for the cryopreservation of natural and engineered tissues. [Google Scholar]
- 34.Merani S. Toso C. Emamaullee J. Shapiro A.M. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95:1449. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- 35.Noth U. Grohn P. Jork A. Zimmermann U. Haase A. Lutz J. 19F-MRI in vivo determination of the partial oxygen pressure in perfluorocarbon-loaded alginate capsules implanted into the peritoneal cavity and different tissues. Magn Reson Med. 1999;42:1039. doi: 10.1002/(sici)1522-2594(199912)42:6<1039::aid-mrm8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann U. Noth U. Grohn P. Jork A. Ulrichs K. Lutz J. Haase A. Non-invasive evaluation of the location, the functional integrity and the oxygen supply of implants: 19F nuclear magnetic resonance imaging of perfluorocarbon-loaded Ba2+-alginate beads. Artif Cells Blood Substit Immobil Biotechnol. 2000;28:129. doi: 10.3109/10731190009118576. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson P.O. Palm F. Andersson A. Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]