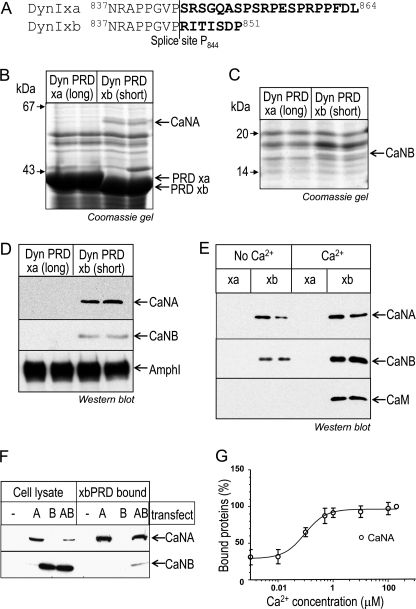
FIGURE 1.
CaN specifically associates with short tailed dynIxb in a Ca2+-regulated manner. A, amino acid sequence alignment of the C terminus of dynIxa (long) and dynIxb (short) splice variants. The amino acids in boldface type are the alternatively spliced sequences. B and C, CaN specifically binds to dynIxb PRD. Synaptosomal lysates were incubated with GST-dynI PRD (either xa or xb) coupled to GSH-Sepharose beads. Bound proteins were separated by SDS-PAGE and stained with Coomassie Blue. The proteins bound to dynIxb PRD at about 58 kDa (B) and about 19 kDa (C) were identified by LC-MSMS as the A and B subunits of protein phosphatase 2B (calcineurin, CaNA, and CaNB). D, bound proteins from pull-down experiments with dynI PRD (xa or xb) were subjected to Western blot analysis with antibodies against CaNA, CaNB, and amphiphysin I. E, binding of CaNA and CaNB subunits to GST-dynIxb PRD is increased in the presence of Ca2+, and CaM binds to dynIxb PRD only in the presence of Ca2+. Proteins from synaptosomal lysate were pulled down by GST-dynI PRD (xa or xb) in the absence or presence of 200 μm Ca2+. CaN or CaM binding was revealed by Western blot. F, CaNB binds dynIxb PRD via CaNA. COS7 cells were transiently transfected with either CaNA or CaNB or cotransfected with both. Cell lysates were incubated with GST-dynIxb PRD coupled to GSH-Sepharose beads. Bound proteins were subjected to Western blot analysis with antibodies against CaNA and CaNB. Left, protein expression in cell lysates; right, proteins bound to dynIxb PRD. G, synaptosomal lysates were used for pull-down experiments with GST-dynIxb PRD in the presence of EGTA (first data point) or various concentrations of Ca2+. Bound proteins were subjected to Western blot with antibodies against CaNA, and the amount of proteins bound was quantified by densitometric analysis of the blots. Data are normalized to 100% against maximum binding in 200 μm Ca2+. All gels and blots are representative of at least three (B–E) or two (G) independent experiments, with some data for gels and blots being shown in duplicate (B–E). Error bars, S.E.