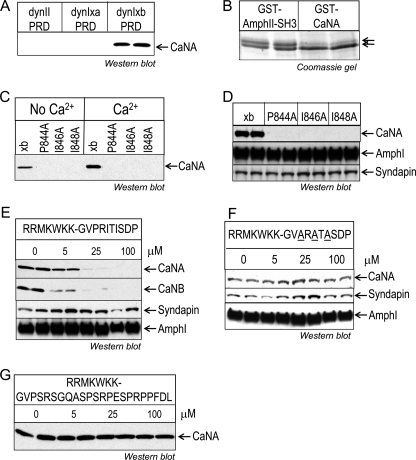
FIGURE 2.
Mapping the CaN binding site within the dynIxb PRD. A, CaN specifically binds to dynIxb PRD but not dynIIxa. Synaptosomal lysates were incubated with GST-dynI PRD (either xa or xb) or GST-dynII PRD coupled to GSH-Sepharose beads, and CaNA binding was revealed by Western blot. B, dynIxb specifically binds to GST-CaNA. Synaptosomal lysates were incubated with either GST-CaNA(2–347) or GST-AmpII-SH3 coupled to GSH-Sepharose beads. Bound proteins were stained with Coomassie Blue after separation by an acrylamide gel with low bis-acrylamide and elevated pH, which is known to resolve the dynI splice variants (27). C, CaN binds to the alternatively spliced C-terminal tail of dynIxb(844–851). Single point mutations were made in the PRITIS motif of dynIxb PRD. The bacterially expressed mutant GST-dynIxb PRDs were used for pull-down experiments from synaptosomal lysates. Bound proteins were subjected to Western blotting with anti-CaNA antibodies. D, mutations in the PRITIS motif do not affect amphiphysin I or syndapin binding to dynIxb PRD. E, peptide competition revealed binding of CaN to the C terminus of dynIxb PRD. The penetratin-tagged peptide mimetic of the short variant, dynIxb(842–851), containing the entire spliced insert was synthesized and used for competition studies. Synaptosomal lysates were incubated with GST-dynIxb PRD immobilized on GSH-Sepharose beads in the absence or presence of 5–100 μm peptide. Binding of proteins was detected by Western blot analysis with antibodies against CaNA, CaNB, syndapin, and amphiphysin I. F, mutant peptide competition determined the specificity of the binding of CaN to the C terminus of dynIxb PRD. The penetratin-tagged mutant peptide containing ARATA was synthesized and used for competition studies. Synaptosomal lysates were incubated with GST-dynIxb PRD immobilized on GSH-Sepharose beads in the absence or presence of 5–100 μm mutant peptide. Binding of proteins was detected by Western blot analysis with antibodies against CaNA, syndapin, and amphiphysin I. G, CaN binding to GST-dynIxb PRD is unaffected by the alternatively spliced C-terminal tail of dynIxa(842–864). The penetratin-tagged peptide dynIxa(842–864) was synthesized and used for a pull-down experiment as in E. CaNA binding to the dynIxb PRD was analyzed by Western blot with anti-CaNA antibodies. All results are representative of two independent experiments. AmphI and AmphII, amphiphysin I and II, respectively.