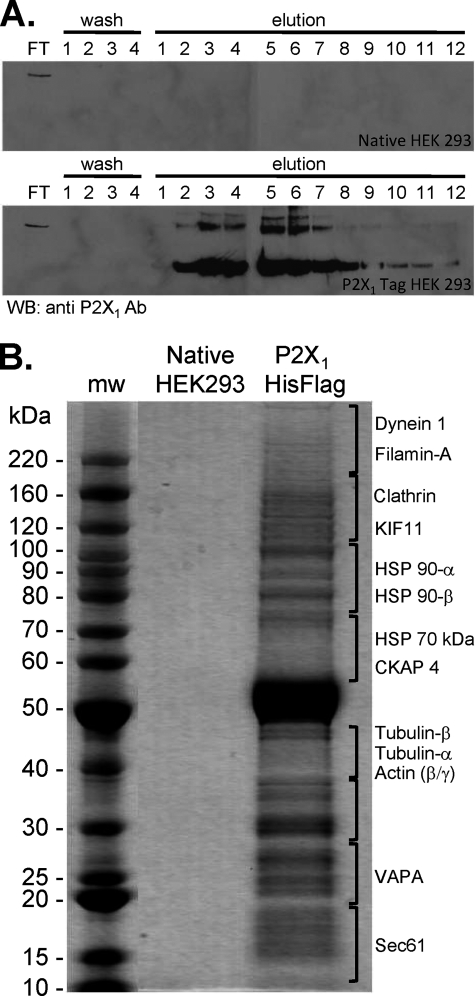
FIGURE 1.
Isolation of P2X1 receptors and associated proteins. A, anti-FLAG antibody-agarose beads were incubated with lysates of either native HEK293 cells or HEK293 cells stably expressing human P2X1 His-FLAG-tagged receptor. The agarose bead-protein complex was loaded onto a column and flow-through (FT) collected. Beads were washed four times and a sample of eluate kept (washes 1–4). 3× FLAG peptide (0.1 mg/ml) eluted 10 1-ml fractions (elutions 1–10) followed by two fractions eluted with 0.1 m glycine (pH 3.5) (elutions 11 and 12). Fractions were analyzed by Western blotting and probed with P2X1 antibody. No immunoreactivity was observed in HEK native cell lysate fractions, but strong immunoreactivity was observed in fractions E2–E8 from HEK293 cells expressing P2X1 receptor. B, fractions E2–E8 were pooled, concentrated, and run on SDS-PAGE after which the gel was stained for the presence of proteins. Cytoskeletal proteins are listed on the right as identified by mass spectrometery from HEK293 cells expressing P2X1 receptor lane gel slices. No proteins were identified from the HEK native receptor lane gel slices.