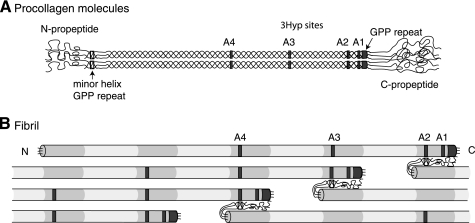
FIGURE 1.
Relative molecular positions of prolyl 3-hydroxylation sites in aligned procollagen molecules and a microfibril. A, occupied 3Hyp sites are indicated by gray boxes within the triple helical regions of the molecule. From right to left, these are the C-terminal (GPP)4 repeat, A1 at Pro-986, A2 at Pro-944, A3 at Pro-707, and A4 at Pro-470. The GPP repeat in the minor triple helix of the N-propeptide is indicated by an open box. B, axial relationships required for trifunctional intermolecular cross-link formation in a pN type II collagen microfibril. Collagen molecules are aligned in the typical collagen D-periodic stagger of 234 amino acid residues. Note the position of the N-propeptide GPP repeat (open box) relative to an A2 site in an adjacent pN type II collagen molecule and alignment of D-staggered A2, A3, and A4 3Hyp sites.