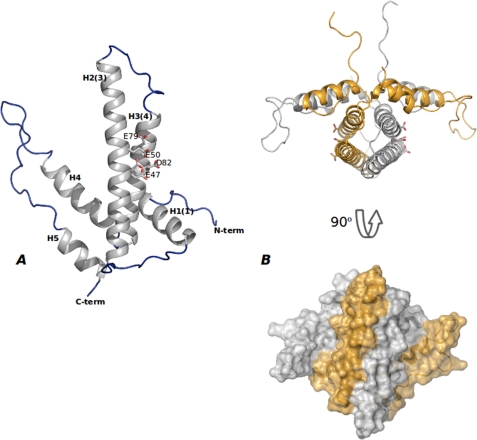
FIGURE 1.
Overall shape of DR2231 protein. A, monomeric DR2231 protein structure. DR2231 is an all-α-helix structure with an overall hairpin-like fold. Secondary structure elements are labeled both according to sequence and to the convention of Moroz et al. (7) (in parenthesis) for facility of comparison with other dUTPase/MazG proteins. The EEXX(E/D) motif is presented (Glu47, Glu50, Glu79, and Asp82); Glu47 appears in a double conformation when in the absence of the divalent metal cation. B, DR2231 dimer in open conformation, presented also in orthogonal view. A surface representation illustrates extension of the interface in the dimer.