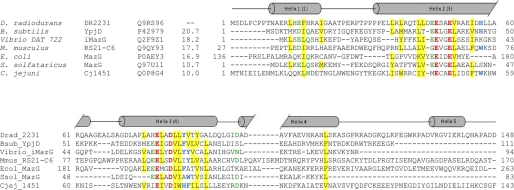
FIGURE 2.
Sequence alignment of DR2231 with members of the dUTPase and MazG subfamilies. The alignment was constructed on the basis of a ClustalW 2.0.12 multiple sequence alignment (58) with minimal manual editing. Experimentally determined crystal structures are available for all listed entries: Bsub_YpjD (PDB code 2GTA), Vibrio_iMazG (PDB codes 2Q5Z and 2Q73), Mmus_RS21-C6 (PDB code 2OIE), Ecol_MazG (PDB code 3CRC), Ssol_MazG (PDB code 1VMG), and Cjej_1451 (PDB code 1W2Y). The proteins are listed by name of the source organism, followed by the protein name; the third column corresponds to UniProt identifiers, and the fourth column shows the percentage of identity with DR2231 sequence. Sequence numbers preceding and following matched segments are indicated. The structural elements shown above the alignment correspond to DR2231 determined in this work. Cylinders indicate α-helices, and the numbering of helices in parenthesis corresponds to the convention established by Moroz et al. (7). The Mg2+-binding residues are shown in red boldface type, and conserved residues are colored as follows: hydrophobic (yellow background), aromatic (blue boldface type), Asp/Asn/Glu/Gln (green boldface type). The E. coli MazG alignment is performed only on the enzymatically active C-terminal domain (residues 136–263); the C. jejuni dUTPase sequence was truncated at residue 143.