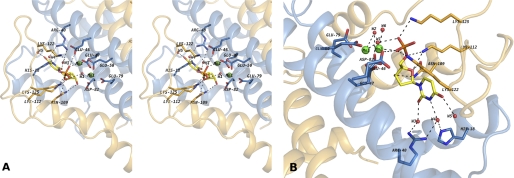
FIGURE 5.
A, stereo view of the putative binding site of DR2231 in the closed conformation bound to dUMP. The active site shows the contribution of residues from both monomers to substrate binding and coordination. When crystallized in presence of Mg2+, DR2231 presents only one divalent metal-coordinated equivalent to Mn1; a water molecule was modeled in the position of Mn2 (not shown). B, close-up of the binding pocket highlighting the role of structured waters in catalysis (W1 and W2), based on homology with C. jejuni dUTPase and substrate binding (W3, W4, and W5). W6 participates in the hexacoordination shell of Mn2.