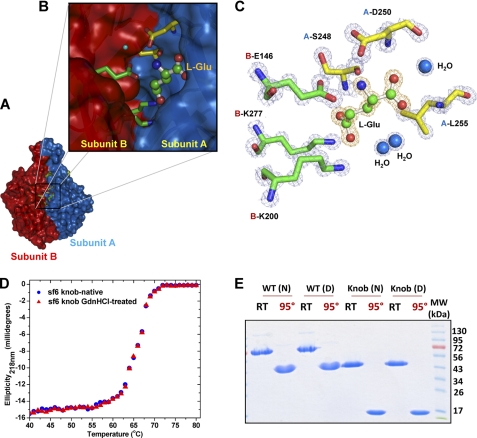
FIGURE 3.
l-Glu binding cleft in Sf6 tail needle knob. A, surface representation of Sf6 knob highlighting the interface between subunits A and B (colored in blue and red, respectively). B, zoom-in view of the subunit interface where l-Glu is bound. Subunit A and B side chains from the final refined model are shown in sticks, and l-Glu is shown in a stick-and-ball representation. C, the final refined model for l-Glu and the interacting residues from both subunits are overlaid to the final 2Fo − Fc electron density map calculated at 1.0 Å resolution and contoured at 1.5σ above background (in blue). The l-Glu is bound in a cleft formed at the interface of two knob monomers, closely surrounded by B-Glu146, B-Lys277, A-Leu255, B-Lys200, A-Ser248, and A-Asp250. D, thermal stability of the native and GdnHCl-treated Sf6 knob. Both samples unfold irreversibly with a melting temperature (Tm) of ∼67 °C. E, SDS resistance assay of wild type Sf6 tail needle and Sf6 knob that were previously treated under native conditions (N) or dialyzed against 1.5 m GdnHCl, 0.5 m NaCl (D). RT and 95° denote samples that were either incubated at 22 °C or boiled for 5 min prior to being electrophoresed.