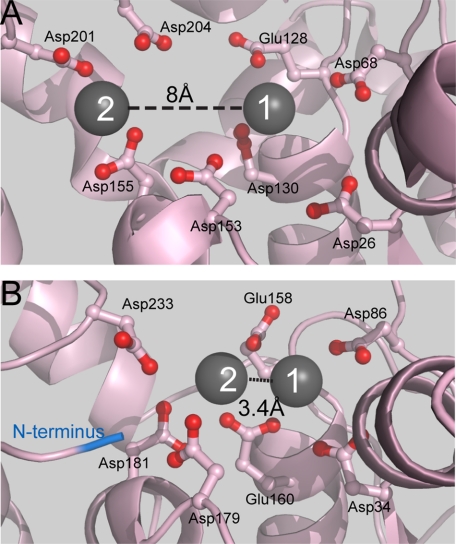
FIGURE 1.
The varying positions of metal ions in FEN structures. A, the active site structure of T5FEN (1UT5) illustrating the seven active site carboxylates present in similar positions in all FENs and the eighth carboxylate (Asp-201) present in the active sites of bacteriophage and bacterial enzymes. Two metal ions, M1 and M2, are bound with a separation of 8 Å. A metal site 2 mutant was created by alteration of the two aspartic acid residues (D210I/D204S). B, the active site structure of hFEN1 (1UL1x), illustrating the seven active site carboxylates present in similar positions in all FENs. Two metal ions, M1 and M2, are bound with a separation of 3.4 Å. Although all FENs conserve seven active site carboxylates, the position of M2 observed in each structure is variable. The N terminus (blue) occupies a similar position to M2 in the bacteriophage enzyme (A).