Abstract
Background:
Idiopatiic pulmonary fibrosis (IPF) is a disease of dysregulated fibrogenesis with abnormal matrix metalloproteinase (MMPs) activity, angiogenesis, and profibrotic milieu wherein MMPs inhibition appears to be target-based therapy. We evaluated the role of doxycycline as a nonspecific inhibitor of MMPs in IPF patients.
Materials and Methods
Patients of IPF diagnosed on the basis of ATS-ERS consensus criteria were put on oral doxycycline in an open prospective trial. They were followed up for long term with spirometry, 6 min walk test (6MWT), St. Georges respiratory questionnaire (SGRQ), forced vital capacity (FVC), and repeat bronchoscopy while on doxycycline monotherapy for over 24 weeks. Both the initial and follow-up broncho alveolar lavage fluids (BALF) from IPF patients (n = 6) and control subjects (n = 6) were looked for MMP-9, -3, tissue inhibitor of metalloproteinase (TIMP)-1 and vascular endothelial growth factor (VEGF) expression. Additionally, doxycycline's action on MMP activities in vitro was tested in BALF of IPF patients.
Results:
Doxycycline intervention showed significant improvement in IPF patients in terms of change in 6MWT, SGRQ, FVC, and quality of life. The level of MMP-9, -3, TIMP-1 and VEGF in the BALF were found significantly higher in the IPF patients compared to the controls while doxycycline therapy reduced those parameters nearer to control value. Doxycycline also showed a significant dose-dependent reduction in the in vitro MMPs activities in BALF.
Conclusion:
Doxycycline shows significant prospect in the treatment of IPF through its anti MMPs activities. This is the first report on a case series of long-term doxycycline monotherapy in IPF patients.
Keywords: Broncho alveolar lavage fluid, doxycycline, idiopathic pulmonary fibrosis, matrix metalloproteinase, vascular endothelial growth factor
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF), a difficult to treat lung disease of unknown etiology characterized by chronic relentless fibrosis[1] and with no available effective treatment. The outcome is dismal with a median survival of only 2-3 years.[2] The condition is also characterized by overexpression of profibrotic cytokines, and a relative deficiency of interferon gamma (INFγ). With the recent paradigm shift of understanding pathogenesis as from inflammation to dysregulated fibrogrnesis, the focus of therapeutic intervention has recommended from the use of steroid and immunosuppressive agents to the prevention of fibrosis. Several trials with different agents like colchicin,[3,4] INF-γ,[5–7] antioxidants,[8,9] pirfenidone,[10,11] and prednizone in combination with azathioprine have shown either little or mild effects that needs further validation. Many newer agents targeting different pathogenetic mechanism have been underway.
Of late, the potential role of a class of zinc-requiring endopeptidases called matrix metalloproteinases (MMPs) in the pathogenesis of IPF has attracted attention.[12] They are secreted in latent proenzyme form and are involved in the remodeling and degradation of extra cellular matrix (ECM). An upregulation of MMPs from the imbalance with their tissue inhibitor of metalloproteinases (TIMPs) can lead to release of growth factors from fibroblasts.[13,14] Therefore, the inhibition of MMPs can be a potential therapy for IPF.[15] The authors have experienced such therapeutic improvement in cases of IPF with doxycycline,[16,17] an agent known for its MMPs inhibitory property with its use being accepted as MMPs inhibitor for periodontal disease by the USFDA.[18]
Prolonged use of doxycycline has been well documented without problem of tolerance or toxicity.[19,20] However, the drug has not been selected so far with a potential therapeutic target in IPF as an inhibitor of MMPs except us.[16,17] Given the poor prognosis of IPF patients, the inadequacy of current treatment options, we performed an open perspective trial of therapy with doxycycline. The aim of the study was to assess in IPF patients, the inhibitory role of doxycycline on MMPs alongside the clinical effect on long-term use.
MATERIALS AND METHODS
Study design
This has been an OPD based open, prospective study where the patients were selected and included from the referral OPD observing a defined protocol being approved by the institutional ethics committee. Since this novel experimental therapeutic trial was not sponsored by anyone, we did not decide any target number of inclusion and kept the endpoint as inhibition of the MMPs activity in the BALF after al least 6 months of therapy with secondary endpoints being clinical improvement in terms of 6 min walk test (6MWT), St. Georges respiratory questionnaire (SGRQ), and, lung functional as forced vital capacity (FVC). We kept a control arm too with subjects without having clinically evident systemic disease and high-resolution computerized tomography (HRCT) chest showing no evidence of any interstitial lung disease.
The study procedure
The inclusion criteria were availability of proper informed written consent, HRCT diagnosis of IPF on agreement of a pulmonologist and a radiologist, male and female patients aged 30–70 years, fulfillment of the major and minor criteria according to the ATS-ERS guideline[1] to diagnose IPF, and FVC > 40% of predicted. The patients with history of exacerbation in past 1 month, having any obvious contraindication for doxycycline including the history of hypersensitivity, inability to meet the demand of the protocol for any reason, significant abnormality in liver or renal function, feature of chronic congestive cardiac failure (supported by echocardiography done whenever felt necessary) and pregnancy of lactating state were excluded.
The selection of the control population was accomplished from the patients who underwent bronchoscopy for evaluation of hemoptysis without any interstitial lung disease from clinical and HRCT chest evaluation. These cases had either no abnormality or minimal localized bronchiectasis in HRCT chest. The broncheo alvelor lavage fluid (BALF) was being taken from the lower lobes of the unaffected lungs in every such case.
Following the suspicion of IPF from clinical examination and chest X-ray (PA view), and upon agreement of the diagnosis of IPF on HRCT as discussed, all the patients underwent the evaluation for the diagnosis of IPF as per the ATS-ERS consensus criteria.[1] Concomitant spirometry examination, 6MWT and evaluation through the SGRQ were also performed. Fiber optic bronchoscopy (FOB) and BALF was performed observing the ATS guideline[1,21] to exclude infection with appropriate microbiological evaluation (routine aerobic culture and AFB smear with culture). The BALF sample was immediately transported to laboratory in cold chain for estimation of the MMP -9, -3, and TIMP-1 and vascular endothelial growth factor (VEGF).
Initiation of the experimental agent doxycycline and the follow-up procedure
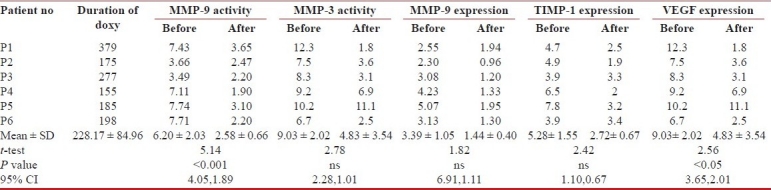
Every patient included in the study received doxycycline orally continuously (100 mg and once or twice daily based on body weight of less then or more than 50 kg) as the sole therapy for IPF alone with other necessary medications for the existing comorbidities. Proper compliance was assured on time to time follow-ups and telephonic enquiry. All the technicians and laboratory colleagues, except the treating physician, were kept blind about the identity, diagnosis, and the follow-up details of the patients. The follow-up were done periodically on a flexible protocol as per the convenience of the patient with clinical, spirometric, radiological (chest X-ray PA view) evaluations along with repeating the 6MWT and SGRQ. Repeat FOB and lavage were performed at any suitable point after at least 24 weeks of therapy with doxycycline. Similarly, we collected the BALF from 6 subjects whom we selected as “control” as described. The demographic details of the cases are given below [Table 1]. All the samples were preserved in fridge (-80°C) for future use.
Table 1.
Effect of doxycycline therapy on BMI, 6MWT, SGRQ and % FVC
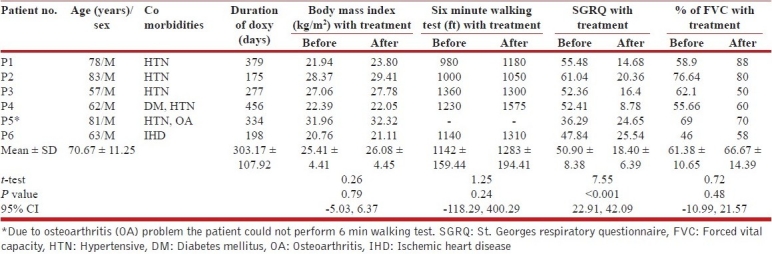
There had been provision of permanent withdrawal and shifting the patient to the conventional therapy under circumstances of no improvement or deterioration at any point of time without any secondary cause (with appropriate evaluation), in any serious adverse events, any significant side effects with the study drug or when the patient show desire to withdraw from the trial.
Assay of MMPs and MMP inhibition
BAL (30 μg protein) from each patient was electrophoresed in SDS-polyacrylamide gel containing either 1 mg/ml gelatin or casein under non-reducing conditions for MMP-9 and MMP-3 assays respectively. Parallel assays were performed on BALF with the addition of 1,10-phenanthroline (150μM in 10% DMSO), doxycycline (50-150μM in milli-Q water) and 10% DMSO and incubation for 1.5 h at 37°C. The gels were washed twice in 2.5% Triton X-100 and then incubated in calcium assay buffer (40 mM Tris-HCl, pH 7.4, 0.2 M NaCl, 10 mMCaCl2) for 18 h at 37°C. Gels were stained with 0.1% Coomassie blue followed by destaining. The zones of gelatinolytic or caseinolytic activities appeared as negative staining. Quantification of zymographic bands was done using densitometry linked to proper software (Lab Image, Kapelan Gmbh, Germany).
Western blotting
BALF (100 μg protein) from each patient was resolved by 8% reducing SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked for 2 h at room temperature in 3% BSA solution in 20 mM Tris-HCl, pH 7.4 containing 150 mM NaCl and 0.02% Tween 20 (TBST) followed by overnight incubation at 4°C in 1:200 dilution of the respective primary antibodies in TBST containing 0.2% BSA followed by extensive washing with TBST and incubation in alkaline phosphatase-conjugated secondary antibody (1:5000) for 2 h. Polyclonal anti-MMP-9, anti-TIMP-1 and anti-VEGF antibodies were purchased from Santa Cruz Biotechnology, USA. The bands were visualized using 5-bromo-4-chloro-3-indolyl phosphate/ nitro blue tetrazolium substrate solution.
Statistical analysis
Data for clinical and biochemical tests were fitted using Sigma plot. Data were represented as the means ± SEM. P<0.05 was accepted as level of significance. The statistical analysis of the data was done using Graph Pad Instat 3 software. Comparison between groups was done using one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls test. The clinical data were analyzed statistically using the paired Student's t-test.
RESULTS
Characteristics of subjects
Six patients of IPF volunteered for the study; their demographic and functional details are shown in Table 1. They have been continuing the drug till the date of data compilation with a mean duration of 303.17 ± 107.92 days. All of them tolerated the drug well. The repeat BAL has been done after a mean period of 228 ± 84.96 days. Only one patient (NM) has been shifted to conventional therapy upon showing some deterioration following the second BAL. One patient with severe knee osteoarthrosis (patient 5, Table 1) was excluded from performing the 6MWT. Doxycycline was well tolerated in all without any significant side effects. The results of the initial and the final visits were thereafter compared statistically using clinical variables as body mass index (BMI), 6MWT, % of FVC and SGRQ. Concomitant assessment in the changes of MMP and TIMP status in BALF has also been done.
Expression of MMP-9, TIMP-1 and VEGF in patients with IPF and effect of doxycycline treatment
BALF collected from IPF patients (n = 6) and then the same after doxycycline treatment (n = 6) were assessed for MMP-9 and -3 activity. Figure 1 shows the positive association of gelatinolytic and caseinolytic activity in BALF of IPF patients. Two major gelatinolytic bands which represented as MMP-9 complex and pro-MMP-9 (92 kDa) were significantly high in IPF patients [Figure 1a]. Pro-MMP-3 activity was also significantly high in IPF patients [Figure 1b].
Figure 1.
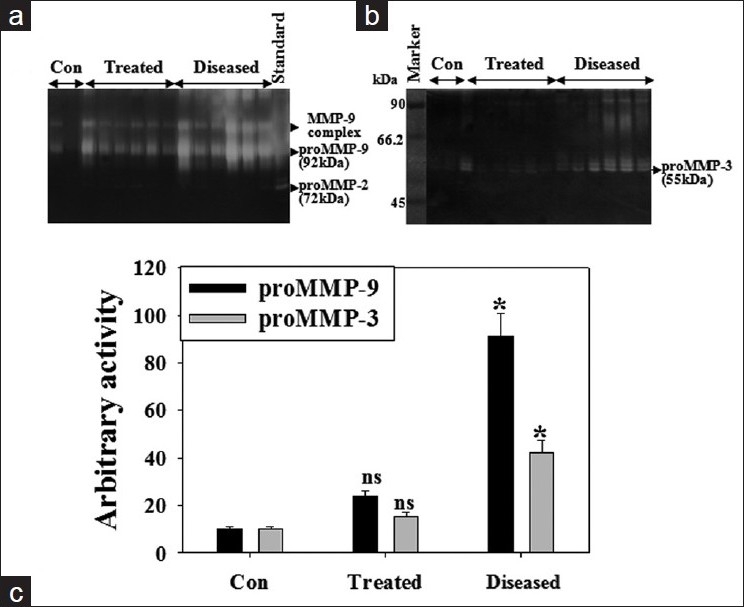
Doxycycline's action on MMP-9 and -3 activities in BALF of IPF patients. Equal amount of BALF protein from control subjects, IPF patients (n = 6) and doxycycline treated IPF patients (n = 6) was subjected to gelatin zymography (a) and casein (b) zymography. Control samples were collected from the noninfected portion of the lungs from IPF patients. Histogram represents proMMP-9 and proMMP-3 activity (c). Values are ± SEM of three zymograms from independent experiments. *P < 0.001 and ns, nonsignificant against an appropriate control
Patient's BALF exhibited higher pro-MMP-9 and -3 activity (~9-fold and ~4-fold in each) as compared to control subjects while doxycycline treatment reversed those enzyme activities near to control level Figure 1c. MMP inhibitors, doxycycline and 1,10-phenanthroline inhibited pro-MMP-9 activity in patient's BALF by 50% and 90% in vitro [Figure 2a–c]. There was a gradual decrease in the activity of pro-MMP-9 as well as MMP-9 complex. Inhibition of pro-MMP-9 activity by doxycycline was dose dependent with its inhibitory role on MMP-9 complex [Figure 2b].
Figure 2.
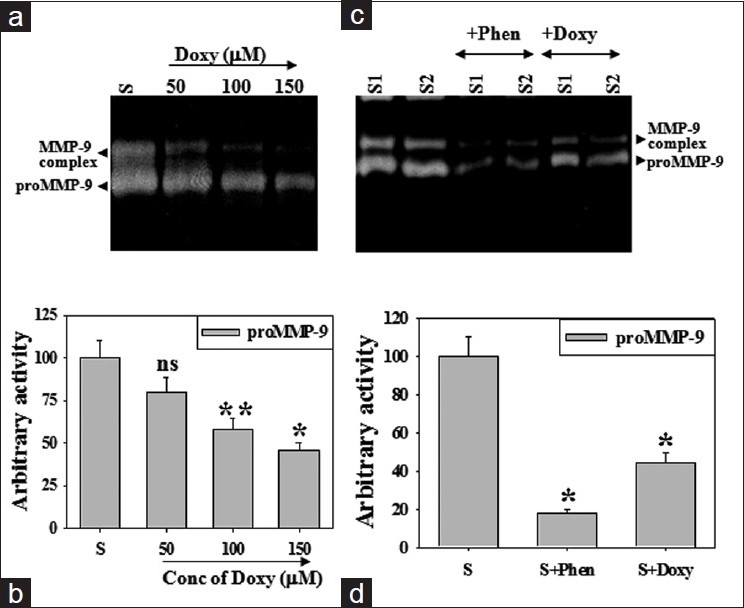
Inhibitory role of doxycycline on MMP-9 activity in vitro. Equal amount of BALF protein from patients with IPF was incubated with increasing concentration of doxycycline and subjected to gelatin zymography (a). Histogram represents pro-MMP-9 activity (b). BALF of IPF patients (S1, S2) was incubated with 1,10-phenantholine or doxycycline and gelatin zymography were performed (c). Histogram represents pro-MMP-9 activity (d). Values are ± SEM of three zymograms from independent experiments. *P < 0.001 and **P < 0.01 while ns, non-significant against an appropriate control
In order to check proteinase/antiproteinase balance in BALF of IPF patients, we performed Western blot for MMP-9 and TIMP-1. Histographic and statistical analyses revealed that both MMP-9 and TIMP-1 expression in patient's BALF was ~8 fold and ~4 fold higher as compared to control ones [Figure 3a]. Doxycycline treatment reduced TIMP-1 expression to control level but MMP-9 expression to ~50% low. The angiogenic marker, VEGF expression was ~5-fold higher in IPF than in control BALF while doxycycline therapy reduced the expression by 50% [Figure 3c]. Correlation of IPF disease outcome and level of MMP-9, -3 and, TIMP-1 as well as VEGF was found [Table 2].
Figure 3.
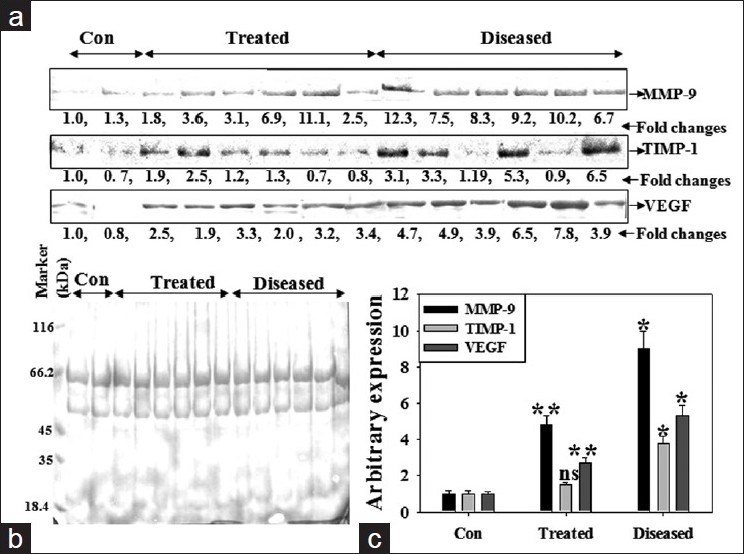
Elevated expression of MMP-9, TIMP-1 and VEGF in patients with IPF. Equal amount of BALF protein from BAL from control subjects, IPF patients and doxycycline treated IPF patients was subjected to Western blotting for MMP-9, TIMP-1 and VEGF (a). Equal loading of BALF protein in each lane was visualized through Ponseu S staining of the blot (b). The intensity of proteins was represented in histogram (c). Values represented in the histogram are ± S.E.M obtained from three independent experiments. *P < 0.001 and **P < 0.01 while ns, non-significant against an appropriate control
Table 2.
Effect of doxycycline therapy on of MMP-9, MMP-3, TIMP-1 and VEGF
DISCUSSION
IPF is not a homogeneous disease rather a multi-etiological condition with multimodal development with poor survival prospect.[22] The pathogenesis appears complex with an unique epithelial/fibroblastic paradigm where a number of important event seemingly play significant role. Among them, the epithelial and fibroblast cell proliferation, tissue protease/antiprotease balance, epithelial mesenchimal transition at the sites of fibroblastic foci, the activation of local pro-coagulant and anti-fibrinolytic activity are important. In addition, there are other important cophenomena as (i) inflammation evident from increased infiltration of macrophages and neutrophils,[23] (ii) intraalveolar coagulopathy,[24] and (iii) abnormal angiogenesis that have been linked to this fibrotic disorders of the lung.[25] The scientific community has been in lookout for a safe, effective and new therapy for such a chronic and progressive fibrotic lung disease.[26] Thus, the research focus is shifted to intervene at the basic and molecular level of mediators to prevent the process of fibrosis.
A coordinated regulation of matrix proteolysis and endothelial cell migration is essential for new blood vessel formation. MMPs, the essential enzyme system for ECM remodeling and angiogenesis has been implicated in the pathogenesis of IPF.[12] They play important role in abnormal alveolar permeability as well since VEGF, a known potent inducer of capillary permeability,[27] acts in part via actions upon MMP.[28,29] Matrix remodeling ensues with very initiation of the disease with a MMPs/TIMPs imbalance favoring over activity of the enzyme to stimulate fibrogenesis.[30,31] The expression of MMP-7 has been high in human IPF and also in the murine models of fibrosis whilst attenuated fibrotic reactions has been observed in MMP-7 knockout mice have.[32,33] Elevated levels of MMP-1,-8,-9 have been found in conjunction with changes in the levels of their soluble inhibitors, TIMPs in IPF. Batimastate, a known MMPs inhibitor has been found to prevent bleomycin induced fibrosis of lungs in mice.[34,35] In view of this, the use of MMPs inhibitors for lung fibrosis in human is just a question of time.
It has been found that the conventional therapy with steroid, azathioprine, and N-acetyl cysteine does not have any effect on the BALF MMPs level and in general the so called standard therapy has limited effects on the course and prognosis of IPF.[15] This observation was prudent despite a previous study suggesting that steroid therapy does reduce MMP-9 production from IPF patients treated with steroids and azathioprine,[24] since in that study patients were not individually studied consecutively pre and post treatment. Most of the new trials with several agents as pirfenidone, colchicine, bosentan, INFγ etc. raised more noise and toxicities than the actual effect.[3–11] Many new agents are already in trial or registered for trial. In this context, trial with a non specific MMPs inhibitor doxycycline is appropriate. The drug is in market for over 30 years, in many long term uses without significant toxicities[19,20] and is cleared for use in periodontal disease as an MMP inhibitor by the USFDA.[18] Doxycycline binds to Ca2+ /Zn2+ at catalytic sites of MMP in vitro and inhibits the enzymatic activity of MMPs.[36] Our protocol is an open non comparative trial of doxycycline to prove its effectiveness and mechanistic role on MMPs inhibition. Before taking up the study we looked for in vitro MMPs inhibition of BAL by doxycycline that showed a dose responsive effectiveness. Gelatinolytic and caseinolytic activities were attenuated in BALF of IPF patients following therapy by doxycycline suggesting its action is via MMPs inhibition property. Thus, the use of doxycycline appears completely ethical and novel.
Our study had both clinical end point as improvement of lung function as FVC, SGRQ, 6MWT, and biochemical end-point as change in MMPs and TIMPs in the BAL fluid. It is noteworthy that the improvement in FVC and the 6MWT were not very significant compared to SGRQ that was highly impressive (P < 0.0001). Since this is an open prospective trial of the first of its kind we decided to continue the drug instead of a fixed point and keep a close look at the clinico-radio-functional developments in the volunteers. None of the patients who continued the drug had any exacerbation during the period of observation. One other patient, not included in the trial who showed initial improvement on doxycycline had an exacerbation after stopping the drug without any information after about 3 months and finally succumbed to it. However, one patient had slight worsening of the VC; he has been stabilized after adding azathioprine and short course steroid to doxycycline. A trial with doxycycline and the conventional therapy and future trials with other agents acting on the different fibrogenic route or immune suppressive pathway will be worthwhile.
This study has several limitations. First our patient population did not all have lung biopsies to prove UIP although they were well characterized according to international guidelines in a specialist clinic. Second, the difference in age between the IPF patients and our healthy controls may be a confounding factor when comparing MMP levels. To address this we examined the relationship between age and MMP levels in IPF and normal controls and found no correlation. Finally, it is important to understand that MMP levels in BALF do not necessarily reflect activity within the interstitium but our results do confirm findings from previous studies. As BALF being the only sample of the alveolar component of the lung's response to disease, it is likely that the altered production of the other MMPs in sites not sampled by BAL can also contribute to the initiation of ECM remodeling in this disease. To conclude, the study is the first of its kind to demonstrate the role of doxycycline to inhibit MMPs in BALF of IPF patients along with significant improvement in SGRQ on prospective trial. Doxycycline merits further scientific evaluation and may find a place in the management of IPF.
Footnotes
Source of Support: AM and SP acknowledge the receipt of Senior Research Fellowship from Council of Scientific and Industrial Research, New Delhi, India. Work is supported by grants NBA2007 of DBT, IAP001 and CLP 261 of NTRF, India
Conflict of Interest: None declared.
REFERENCES
- 1.American Thoracic Society/European Respiratory Society. International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias.This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 3.Douglas WW, Ryu JH, Swensen SJ, Offord KP, Schroeder DR, Caron GM, et al. Colchicine versus prednisone in the treatment of idiopathic pulmonary fibrosis. A randomized prospective study. Members of the Lung Study Group. Am J Respir Crit Care Med. 1998;158:220–5. doi: 10.1164/ajrccm.158.1.9709089. [DOI] [PubMed] [Google Scholar]
- 4.Selman M, Carrillo G, Salas J, Padilla RP, Perez-Chavira R, Sansores R, et al. Colchicine, D-penicillamine, and prednisone in the treatment of idiopathic pulmonary fibrosis: A controlled clinical trial. Chest. 1998;114:507–12. doi: 10.1378/chest.114.2.507. [DOI] [PubMed] [Google Scholar]
- 5.Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341:1264–9. doi: 10.1056/NEJM199910213411703. [DOI] [PubMed] [Google Scholar]
- 6.Abdelaziz MM, Samman YS, Wali SO, Hamad MM. Treatment of idiopathic pulmonary fibrosis: Is there anything new? Respirology. 2005;10:284–9. doi: 10.1111/j.1440-1843.2005.00712.x. [DOI] [PubMed] [Google Scholar]
- 7.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–33. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 8.Behr J, Degenkolb B, Krombach F, Vogelmeier C. Intracellular glutathione and bronchoalveolar cells in fibrosing alveolitis: effects of N-acetylcysteine. Eur Respir J. 2002;19:906–11. doi: 10.1183/09031936.02.00204902. [DOI] [PubMed] [Google Scholar]
- 9.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 10.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: Results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159:1061–9. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 11.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 12.Henry MT, McMahon K, Mackarel AJ, Prikk K, Sorsa T, Maisi P, et al. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-1 in sarcoidosis and IPF. Eur Respir J. 2002;20:1220–7. doi: 10.1183/09031936.02.00022302. [DOI] [PubMed] [Google Scholar]
- 13.Selman M, Ruiz V, Cabrera S, Segura L, Ramirez R, Barrios R, et al. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis.A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol. 2000;279:L562–74. doi: 10.1152/ajplung.2000.279.3.L562. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JA., Jr Pulmonary fibrosis: Pathways are slowly coming into light. Am J Respir Cell Mol Biol. 2000;22:520–3. doi: 10.1165/ajrcmb.22.5.f185. [DOI] [PubMed] [Google Scholar]
- 15.McKeown S, Richter AG, O’Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J. 2009;33:77–84. doi: 10.1183/09031936.00060708. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya P, Nag S, Ghosh D, Roy Chowdhury S, Bardhan S, Mukherjee A. Treatment of probable idiopathic pulmonary fibrosis with long term doxycycline, a matrix metalloproteinase Inhibitor. Indian J Chest Dis Allied Sci. 2007;49:180. [Google Scholar]
- 17.Bhattacharyya P, Nag S, Bardhan S, Acharyya D, Paul R, Dey R, et al. The role of long term doxycycline in patients of idiopathic pulmonary fibrosis: The results of an open prospective trial. Lung India. 2009;26:81–5. doi: 10.4103/0970-2113.53231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 19.Calza L, Attard L, Manfredi R, Chiodo F. Doxycycline and chloroquine as treatment for chronic Q fever endocarditis. J Infect. 2002;45:127–9. doi: 10.1053/jinf.2002.0984. [DOI] [PubMed] [Google Scholar]
- 20.Ginns LC, Roberts DH, Mark EJ, Brusch JL, Marler JJ. Pulmonary capillary hemangiomatosis with atypical endotheliomatosis: Successful antiangiogenic therapy with doxycycline. Chest. 2003;124:2017–22. doi: 10.1378/chest.124.5.2017. [DOI] [PubMed] [Google Scholar]
- 21.Honeybourne D, Babb J, Bowie P, Brewin A, Fraise A, Garrard C, et al. (British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of the Standards of Care Committee of the British Thoracic Society). British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56:i1–21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: Multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–9. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]
- 23.Kinder BW, Brown KK, Schwarz MI, Ix JH, Kervitsky A, King TE., Jr Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest. 2008;133:226–32. doi: 10.1378/chest.07-1948. [DOI] [PubMed] [Google Scholar]
- 24.Gunther A, Mosavi P, Ruppert C, Heinemann S, Temmesfeld B, Velcovsky HG, et al. Enhanced tissue factor pathway activity and fibrin turnover in the alveolar compartment of patients with interstitial lung disease. Thromb Haemost. 2000;83:853–60. [PubMed] [Google Scholar]
- 25.Cosgrove GP, Brown KK, Schiemann WP, Serls AE, Parr JE, Geraci MW, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: A role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170:242–51. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 26.Antoniou KM, Pataka A, Bouros D, Siafakas NM. Pathogenetic pathways and novel pharmacotherapeutic targets in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2007;20:453–61. doi: 10.1016/j.pupt.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Thickett DR, Armstrong L, Christie SJ, Millar AB. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1601–5. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- 28.Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3’-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–9. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 29.Jin HY, Lee KS, Jin SM, Lee YC. Vascular endothelial growth factor correlates with matrix metalloproteinase-9 in the pleural effusion. Respir Med. 2004;98:115–22. doi: 10.1016/j.rmed.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, et al. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–8. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 31.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–51. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 32.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuorinen K, Myllarniemi M, Lammi L, Piirila P, Rytila P, Salmenkivi K, et al. Elevated matrilysin levels in bronchoalveolar lavage fluid do not distinguish idiopathic pulmonary fibrosis from other interstitial lung diseases. Apmis. 2007;115:969–75. doi: 10.1111/j.1600-0463.2007.apm_697.x. [DOI] [PubMed] [Google Scholar]
- 34.Corbel M, Caulet-Maugendre S, Germain N, Molet S, Lagente V, Boichot E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J Pathol. 2001;193:538–45. doi: 10.1002/path.826. [DOI] [PubMed] [Google Scholar]
- 35.Fujita M, Ye Q, Ouchi H, Harada E, Inoshima I, Kuwano K, et al. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob Agents Chemother. 2006;50:739–43. doi: 10.1128/AAC.50.2.739-743.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160:1089–95. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


