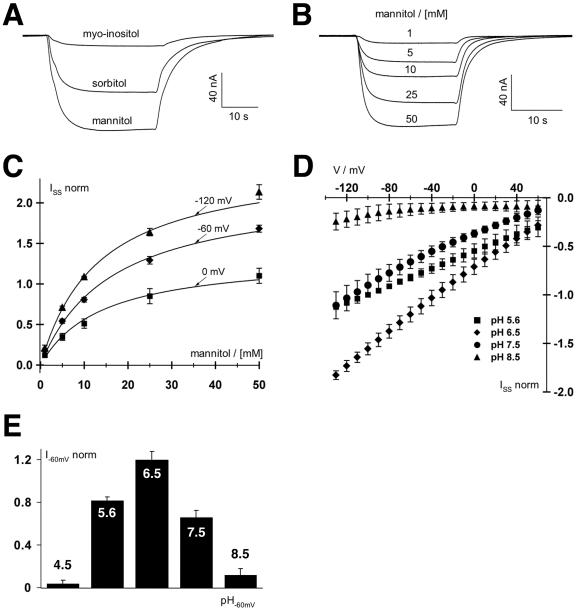
Figure 6.
Biophysical analyses of PmPLT1 expressed in Xenopus sp. oocytes with the double-electrode voltage clamp technique. A, Polyol-induced inward H+ currents mediated by PmPLT1-injected oocytes. Increase in H+ currents in response to 30-s pulses of 30 mm mannitol, sorbitol, or myoinositol at pH 5.6 at a holding potential of -60 mV. Only mannitol and sorbitol but not myoinositol are substrates of PmPLT1. Relative currents were: Imannitol = 1, Isorbitol = 0.66 ± 0.11, and Imyoinositol = 0.12 ± 0.05 (data points were normalized to the currents at -60 mV in 30 mm mannitol; mean ± sd, n = 4). B, Whole-cell currents through PmPLT1 in response to a stepwise increase of mannitol concentration at pH 5.6 and a holding potential of -60 mV. C, Michaelis-Menten kinetics of PmPLT1 for mannitol at membrane potentials of 0, -60, and -120 mV. Data points represent the mean ± sd of five independent experiments normalized to the currents at -100 mV in 10 mm mannitol. Currents in the absence of mannitol were subtracted for leak correction. Continuous lines show the best nonlinear regression fits of the data points to the Michaelis-Menten equation. D and E, Mannitol-induced currents of oocytes injected with PmPLT1 cDNA are voltage and pH dependent. D, Whole-cell steady-state currents (ISS) in response to 10-mV voltage steps from 60 to -130 mV were recorded upon perfusion with 10 mm mannitol solutions at varying pH values from 4.5 to 8.5, as indicated. E, The pH optimum of PmPLT1 (determined at -120 mV) is 6.5. Data points represent mean ± sd, n = 4. Normalization to the currents at -100 mV in pH 5.6 and leak subtraction were performed as in C.