Table 3.
Preparative Palladium-Catalyzed Allylations of Substituted Aromatic Bromides Using (Z)-1b and 3a
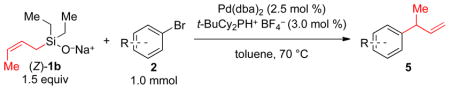 | |||
|---|---|---|---|
| entry | product | yield,b % | γ/αc |
| 1 |
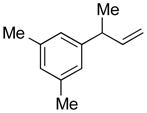 5a |
90 | >99:1 |
| 2 |
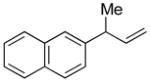 5b |
92 | >99:1 |
| 3 |
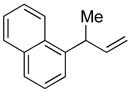 5c |
89 | >99:1 |
| 4 |
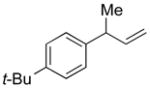 5d |
94 | >99:1 |
| 5 |
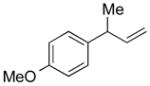 5e |
85 | >99:1 |
| 6 |
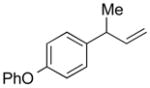 5f |
84 | >99:1 |
| 7 |
5g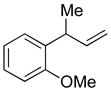
|
85 | >99:1 |
| 8 |
5h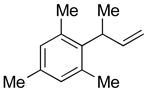
|
73 | >99:1 |
| 9 |
5i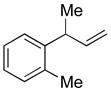
|
74 | >99:1 |
| 10d |
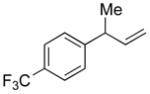 5j |
82 | >99:1 |
| 11 |
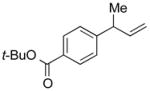 5k |
91 | >99:1 |
| 12 |
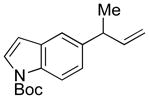 5l |
88 | 99:1e |
| 13d,f |
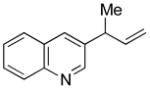 5m |
82 | 25:1 |
| 14 |
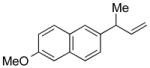 5n |
91 | >99:1 |
Reactions performed on 1.0 mmol scale.
Yield of isolated, purified product.
GC peak area ratio of crude reaction mixture.
5% Pd(dba)2 and 6% t-BuCy2PH+BF4− used.
Determined by 1H NMR analysis (>100:1 S/N).
90 °C reaction temperature used.
