Abstract
Objectives
Reports of an association between antineutrophil cytoplasmic antibodies (ANCA) and autoimmune neutropenia have rarely included cases of proven vasculitis. A case of ANCA-associated vasculitis (AAV) with recurrent neutropenia is described and relevant literature on the association between ANCA, neutropenia, and vasculitis is reviewed.
Methods
Longitudinal clinical assessments and laboratory findings are described in a patient with AAV and recurrent episodes of profound neutropenia from December 2008 – October 2010. A PubMed database search of the medical literature was performed for papers published from 1960 through October 2010 to identify all reported cases of ANCA and neutropenia.
Results
A 49 year-old man developed recurrent neutropenia, periodic fevers, arthritis, biopsy-proven cutaneous vasculitis, sensorineural hearing loss, epididymitis, and positive tests for ANCA with specificity for antibodies to both proteinase 3 and myeloperoxidase. Antineutrophil membrane antibodies were detected during an acute neutropenic phase and were not detectable in a post-recovery sample, whereas ANCA titers did not seem to correlate with neutropenia. An association between ANCA and neutropenia has been reported in 74 cases from 24 studies in the context of drug/toxin exposure, underlying autoimmune disease, or chronic neutropenia without underlying autoimmune disease. In these cases, the presence of atypical ANCA patterns and other antibodies were common; however, vasculitis was uncommon and when it occurred was usually limited to the skin and in cases of underlying toxin exposure.
Conclusions
ANCA is associated with autoimmune neutropenia, but systemic vasculitis rarely occurs in association with ANCA and neutropenia. The interaction between neutrophils and ANCA may provide insight into understanding both autoimmune neutropenia and AAV.
Keywords: vasculitis, neutropenia, antineutrophil cytoplasmic antibody (ANCA)
INTRODUCTION
Autoimmune neutropenia is defined as a circulating absolute neutrophil count (ANC) of less than 1500/μL due to an immune-mediated process. Primary autoimmune neutropenia occurs in the absence of any other detectable pathology and is usually a benign condition seen in newborns. Secondary autoimmune neutropenia occurs in cases of underlying malignancy, infection, toxic exposure, or autoimmune disease including Felty's syndrome, systemic lupus erythematosis, and large granular lymphocyte syndrome (1). Antineutrophil membrane antibodies, directed against antigens located on the neutrophil cell surface, are recognized causes of both primary and secondary autoimmune neutropenia (1). In contrast, despite neutrophil antigens being the antigenic target of antineutrophil cytoplasmic antibodies (ANCA), neutropenia is not typically associated with ANCA or ANCA-associated diseases.
A case of recurrent neutropenia in a patient with ANCA who subsequently developed ANCA-associated vasculitis (AAV) is described, and the existing medical literature on the potential association between ANCA, autoimmune neutropenia, and vasculitis is reviewed.
METHODS
Longitudinal clinical and laboratory assessments were performed in a case of AAV and recurrent neutropenia. Relevant information was excerpted from medical records and contemporaneous interviews with the patient. Serial assessments for ANCA and antineutrophil membrane antibodies were performed during periods of neutropenia and recovery. Testing was performed for both cytoplasmic (c) and perinuclear (p) ANCA by indirect immunofluorescence, and enyzme-linked immunosorbent assays (ELISA) were used to test for antibodies to proteinase 3 (PR3) and myeloperoxidase (MPO) (2). Granulocyte-reactive antibodies directed against neutrophil membrane antigens were measured using a modified indirect granulocyte immunofluorescence test (GIFT) (3).
A PubMed search of the medical literature for 1960 through October 2010 was performed using the following subject heading terms and keywords: [leukopenia OR leucopenia OR neutropenia OR agranulocytosis] and [systemic vasculitis OR antineutrophil cytoplasmic antibodies OR antineutrophil cytoplasmic antibody vasculitis OR ANCA OR vasculitis]. Bibliographies of identified reports and review articles were hand searched for additional references. Only pertinent literature, primarily in the English language, was included. Articles describing neutropenia as a side effect of therapy in cases of AAV with medications known to cause neutropenia (e.g. cyclophosphamide, rituximab, azathioprine) were excluded from further review.
RESULTS
A case of AAV and recurrent neutropenia with disease manifestations including periodic fevers, arthritis, biopsy-proven cutaneous vasculitis, sensorineural hearing loss, epididymitis, and positive tests for antineutrophil membrane antibodies and ANCA with specificity for antibodies to both PR3 and MPO is described.
CASE REPORT
In June 2008, a previously healthy 49 year-old man developed a distinctive pattern of high-spiking fevers (temperature >39°C), arthralgias, and fatigue. On day 1 of his typical symptom cycle, he had progressive fatigue. On day 2, he had fevers, shaking chills, and symmetric arthralgias. On day 3, these symptoms were most severe. On day 4, he gradually recovered. On days 5–7, he felt well.
The patient was hospitalized in July 2008 for evaluation of fever and found to be neutropenic with a total white blood cell count of 2300/μL and an absolute neutrophil count (ANC) of 0/μL. A bone marrow biopsy revealed normal marrow precursors, maturation arrest of myeloid elements, and no malignancy. The neutropenia spontaneously resolved and no explanation for the fevers was identified.
For the next several months, the patient had recurrent fevers, arthralgias, neutropenia, and he always recovered spontaneously. His symptoms usually improved with acetaminophen, and he never received colony stimulating factors for treatment of neutropenia. In August 2008 while neutropenic, an arthrocentesis performed on a painful left ankle yielded inflammatory synovial fluid (15,000 white blood cells; 73% polymorphonuclear leukocytes, 11% histiocytes, 6% lymphocytes) with no crystals detected. In November 2008, his ANC again trended down to 0/μL before spontaneous recovery.
The patient's past medical history was notable for longstanding hypertension that was well-controlled with diltiazem. He took no other medications or supplements. There was no family history of autoimmune diseases or periodic fever syndromes. He lived in Massachusetts, USA and had not recently traveled out of state. He worked in a managerial position. He had a 30-year history of tobacco use but had quit smoking cigarettes several years ago. He reported limited alcohol intake and had no history of illicit drug use, including cocaine.
In December 2008, he was again hospitalized for evaluation of fever and arthralgias. At admission, he was febrile (103° F) and tachycardic. The conjunctivae were injected. Fingers, wrists, elbows, and shoulders were tender without effusions. There was no splenomegaly or lymphadenopathy. Cutaneous, sinonasal, cardiopulmonary, gastrointestinal, neurologic, and genitourinary exams were unremarkable. The WBC at admission was 7500/μL (55% polymorphonuclear leukocytes, 36% lymphocytes, 7% monocytes, 2% eosinophils). ANC was 4125/μL but subsequently fell to 0/μL on hospital day 7 and spontaneously normalized by day 14. Tests for autoimmune and infectious diseases, and for genetic periodic fever syndromes, were negative (Table 1). A bone marrow biopsy during a period of absolute neutropenia again revealed maturation arrest of myeloid elements. Flow cytometry of marrow and peripheral blood did not demonstrate a clonal lymphocyte population.
Table 1.
Selected laboratory tests in current case
| Test | Value | Normal Range |
|---|---|---|
| Tests for autoimmune diseases | ||
|
| ||
| Antinuclear antibody | Negative | Negative |
| Rheumatoid factor (IU/mL) | <20 | <20 |
| Complement component 3 (C3) (g/L) | 0.92 | 0.80–1.70 |
| Complement component 4 (C4) (g/L) | 0.15 | 0.16–0.40 |
| C-reactive protein (mg/L) | 153 | <10 |
| Erythrocyte sedimentation rate (mm/hr) | 98 | 0–15 |
| IgG (g/L) | 17.20 | 7.00–16.00 |
| IgA (g/L) | 9.21 | 0.70–4.00 |
| IgM (g/L) | 23.60 | 0.46–3.04 |
| Serum immunofixation | No monoclonal bands | No monoclonal bands |
|
| ||
| Tests for infectious diseases | ||
|
| ||
| Blood cultures (bacterial, fungal) | Negative | Negative |
| Human immunodeficiency virus (HIV) | Non-reactive | Non-reactive |
| Rapid plasma reagin (RPR) | Non-reactive | Non-reactive |
| Anti-Steptolysin O titer (IU/ml) | <25 | <125 |
| Hepatitis B surface antigen | Negative | Negative |
| Hepatitis C antibody | Negative | Negative |
| Parvovrius B19 (PCR) | Negative | Negative |
| Babesiosis (PCR) | Negative | Negative |
| Ehrlichiosis (PCR) | Negative | Negative |
| Lyme Disease (Antibody, PCR) | Negative | Negative |
|
| ||
| Tests for genetic diseases | ||
|
| ||
| Familial Mediterranean Fever (FMF) | No MEFV gene mutations | No mutations |
| TNF Recepter-1 Associated Periodic Syndromes (TRAPS) | No TNFR1 gene mutations | No mutations |
| Cryopyrin-Associated Periodic Syndromes (CAPS) | No CIAS 1 gene mutations | No mutations |
A diagnosis of vasculitis was considered. Testing for ANCA was positive for moderate titers of antibodies to both PR3 and MPO with a perinuclear immunofluorescence staining pattern. Magnetic resonance imaging of the chest and abdomen, computerized tomography of sinuses, urinalysis, and ophthalmologic exam, revealed no evidence of vasculitis.
In January 2009, he presented with fevers, arthralgias, and purple macules on his right leg. Skin biopsy was consistent with leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrate with leukocytoclasia and extravasated erythrocytes. Treatment for AAV was initiated with high-dose glucocorticoids and oral methotrexate (20mg/week) with rapid resolution of the rash, fevers, and arthralgias.
In May 2009 the patient presented with acute right-sided hearing loss. An audiogram, when compared to a baseline audiogram obtained in March 2009, demonstrated a new right-sided 10–15 decibel sensorineural hearing loss across all frequencies. Treatment included re-initiation of high-dose glucocorticoids, discontinuation of methotrexate, and initiation of oral cyclophosphamide (1mg/kg/day). A subsequent audiogram (June 2009) showed a return to baseline hearing threshold. During treatment with cyclophosphamide, he had only one episode of mild transient neutropenia. In October 2009 the cyclophosphamide was discontinued and azathioprine was started. In late October 2009 the patient developed pancreatitis which resolved when azathioprine was discontinued.
In December 2009 mycophenolate mofetil (2000mg/day) was started. Two weeks later, the patient had severe bilateral testicular pain; ANC was 0.1K/μL. Scrotal ultrasound showed bilateral enlargement of the epididymes with hyperemia and cyst formation consistent with epididymitis. He was treated for 3 days with broad-spectrum antibiotics without improvement. Prednisone 60mg daily was then started with resolution of symptoms and recovery of neutrophil count within several days. Mycophenolate mofetil was discontinued, and he was treated with rituximab (375mg/m2; weekly infusions × 4 doses) (4).
As of October 2010, no further episodes of neutropenia or active manifestations of vasculitis with the exception of arthralgias requiring low doses of prednisone (5–10mg daily) have been noted. ANCA titers for PR3 and MPO have remained persistently positive in low titers. He was treated with a second course of rituximab (375mg/m2; weekly infusions × 4 doses) six months after initial treatment for maintenance of disease remission.
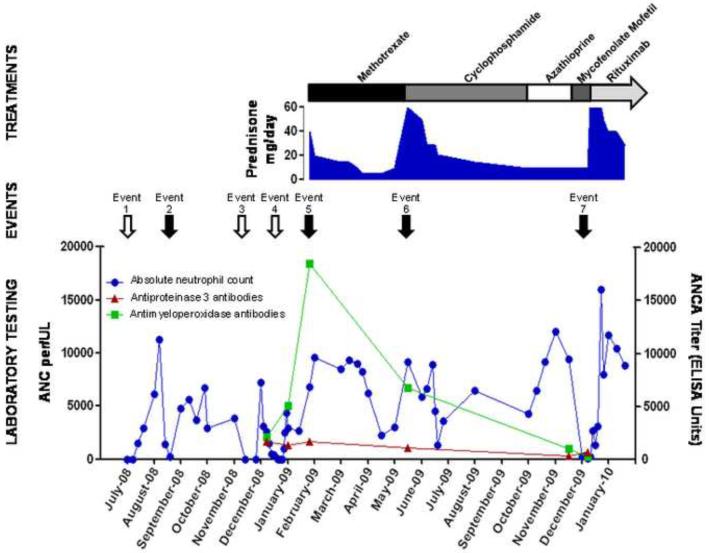
Serial ANCA measurements from December 2008 – December 2009 confirmed the persistence of ANCA with dual specificity to PR3 and MPO. A summary timeline of events, treatment strategies, neutrophil counts, and ANCA titers is provided (Figure 1).
Figure 1. Clinical events, serologic findings, and treatment strategies.
Events 1, 3, and 4 (open arrows): fever, arthralgias, and absolute neutropenia. Events 2, 5, 6, and 7 (closed arrows): manifestations of ANCA-associated vasculitis.
Event 2: inflammatory ankle effusion and neutropenia.
Event 5: cutaneous leukocytoclastic vasculitis, fevers, and arthralgias.
Event 6: sensorineural hearing loss.
Event 7: epididymitis, fever, and absolute neutropenia.
Anti-proteinase 3 titers ranged from 359–1689 units (normal< 2.8 units).
Anti-myeloperoxidase titers ranged from 250–18452 units (normal< 20 units).
ANC = absolute neutrophil count; ANCA = antineutrophil cytoplasmic antibodies.
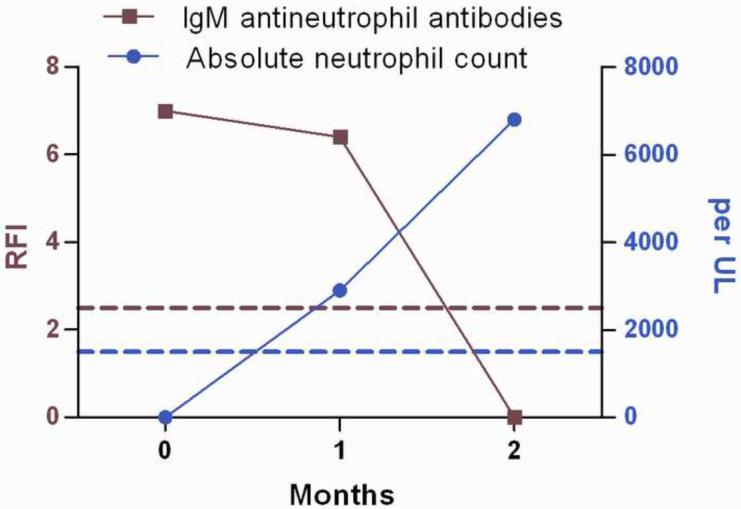
To test for other potential autoimmune causes of neutropenia, granulocyte-reactive antibodies directed against neutrophil membrane antigens were measured using a modified indirect granulocyte immunofluorescence test (GIFT). IgM antineutrophil membrane antibodies were detectable during an acute neutropenic phase and were not detectable in the second post-recovery sample (Figure 2).
Figure 2. IgM antineutrophil membrane antibodies at time of absolute neutropenia (December 2008) and the subsequent two month period during which neutropenia resolved.
Antineutrophil antibody results are expressed as ratios of median fluorescence intensity (RFI) of patient serum-sensitized target cells over median fluorescence intensity of cells incubated in normal control serum. A ratio of ≥ 2.5 is considered positive for IgM neutrophil reactive antibodies (above red dashed line). Neutropenia is defined as an absolute neutrophil count < 1500/UL (below blue dashed line). Antibody testing was done courtesy of the Blood Center of Wisconsin Platelet and Neutrophil Immunology Laboratory.
LITERATURE REVIEW
The literature regarding a possible association between ANCA and neutropenia is mostly limited to case reports and small case series. The search identified 24 articles that described an association between ANCA and neutropenia in a total of 74 patients. Cases reported to date can be categorized into three different clinical scenarios: cases of drug/toxin exposure, cases that occurred in the context of active autoimmune disease, and cases of chronic neutropenia without underlying autoimmune disease. Patient-level data are summarized in Table 2. Among all included studies, female prevalence was 79% and mean patient age was 42 years (range 8–70 years). Reported ANCA immunofluorescence patterns were PANCA 38%, atypical-ANCA 37%, and C-ANCA 25%. ELISA testing demonstrated ANCA specificity to lactoferrin 22%, MPO 18%, unidentifiable antigen 15%, multiple antigens 5%, PR3 4%, and human neutrophil elastase 0.1%; testing for antigen specificity was not described in 35% of cases.
Table 2.
Literature review of association between ANCA and neutropenia
| Reference | Attributed Cause |
Subject Age, Sex |
Degree of Neutropenia |
ANCA (IF) | ANCA (ELISA) |
Signs of Vasculitis |
Antineutrophil Membrane Antibodies |
ANA | Other |
|---|---|---|---|---|---|---|---|---|---|
| Suspected Drug-Induced | |||||||||
|
| |||||||||
| Sera 2000 (6) | Propylthiouriacil | 46,F | ND | p-ANCA | MPO | Purpura | ND | ND | |
| 28,F | ND | p-ANCA | MPO | None | ND | ND | |||
| 33,F | ND | p-ANCA | MPO | None | ND | ND | |||
|
| |||||||||
| Noh 2001 (45) | Propylthiouriacil | 22,F | Mild | p-ANCA | MPO | None | ND | + | |
|
| |||||||||
| Akamizu 2002 (7) | Propylthiouriacil | 45,F | Severe | p-ANCA | MPO,PR3 | None | ND | + | Hypercellular marrow |
|
| |||||||||
| Yamada 2002 (8) | Propylthiouriacil | 13,F | ND | p-ANCA | MPO | Hematuria | + | ND | |
|
| |||||||||
| Farah 2006 (10) | Propylthiouriacil | 70,F | Severe | p-ANCA | ND | Skin-LCV* | ND | ND | |
|
| |||||||||
| Finucane 2008 (37) | Propylthiouriacil | 31,F | Moderate | c-ANCA | PR3 | None | ND | ND | Hypercellular marrow |
|
| |||||||||
| Ozkok 2009 (9) | Propylthiouriacil | 36,F | Moderate | p-ANCA | MPO | Skin-LCV* | ND | − | Splenomegaly Plasmacytosis |
|
| |||||||||
| Kawachi 1995 (11) | Methimazole | 24,F | Moderate | p-ANCA | MPO | LCV* | ND | + | |
|
| |||||||||
| Sangala 2010 (13) | Hydralazine | 55,F | Severe | p-ANCA | MPO | Pauci-immune GN*; Skin-LCV* | ND | + | Hypercellular marrow |
|
| |||||||||
| Ahmed 2008 (12) | Minocycline | 18,M | Mild | c-ANCA | PR3 | None | ND | + | Maturation arrest |
|
| |||||||||
| Knowles 2009 (15) | Cocaine/Levamisole | 4 Cases# | Severe | p-ANCA (2) | ND | ND | ND | ND | |
| c-ANCA (2) | |||||||||
|
| |||||||||
| Bradford 2010 (17) | Cocaine/Levamisole | 39,F | Mild | a-ANCA | HNE | Skin-LCV* | ND | + | LAC |
| 49,F | Severe | a-ANCA | MPO,PR3,HNE | None | ND | + | |||
|
| |||||||||
| Czuchlewski 2010 (18) | Cocaine/Levamisole | 45,F | Severe | ND | ND | None | − | ND | Plasmacytosis |
| 37,M | Severe | None | + | Plasmacytosis | |||||
| 35,F | Severe | None | ND | Plasmacytosis | |||||
|
| |||||||||
| Walsh 2010 (16) | Cocaine/Levamisole | 57,F | Severe | p-ANCA | ND | None | ND | ND | ACA |
| 22,F | Moderate | p-ANCA | ND | Skin-LCV* | ND | ND | ACA | ||
|
| |||||||||
| Barbano 1999 (20) | Levamisole | 8,M | ND | p-ANCA | ND | None | ND | − | ACA, Splenomegaly |
|
| |||||||||
| Underlying Autoimmune Disease | |||||||||
|
| |||||||||
| Juby 1992 (21) | Felty's Syndrome | 11 cases# | Mild-Severe | p-ANCA (4) | ND | ND | ND | ND | Splenomegaly (7) |
| c-ANCA (7) | |||||||||
|
| |||||||||
| Coremans 1993 (22) | Felty's Syndrome | 23 cases# | Mild-Severe | a-ANCA (22) | Lactoferrin (15) MPO (4) Not identified (4) |
ND | ND | + (17) | |
| p-ANCA (1) | − (6) | ||||||||
|
| |||||||||
| Wehbe 2010 (24) | Autoimmune hepatitis | 49,F | Severe | a-ANCA | ND | None | + | ND | Maturation arrest |
|
| |||||||||
| Cuadrado 2009 (25) | Autoimmune hepatitis | 68,F | Severe | p-ANCA | ND | None | − | + | Maturation arrest |
|
| |||||||||
| Hanawa 2010 (26) | Primary Sclerosing Cholangitis | 19,M | Severe | c-ANCA | ND | None | + | + | |
|
| |||||||||
| Jani 2002 (36) | Ulcerative Colitis | 61,F | Moderate | p-ANCA | MPO,PR3 | None | − | + | Splenomegaly |
|
| |||||||||
| Krishnan 1997 (28) | Sjöogren's Syndrome | 52,F | Severe | ND | ND | Skin-LCV | + | + | Maturation arrest |
|
| |||||||||
| Coppo 2004 (27) | Sjöogren's Syndrome | 62,F | Severe | p-ANCA | MPO | None | − | + | Maturation arrest, LGLS |
| 54,F | Severe | p-ANCA | Not Identified | None | − | + | |||
|
| |||||||||
| Present Report | ANCA-Associated Vasculitis | 49,M | Severe | p-ANCA | MPO,PR3 | Skin-LCV*, SN hearing loss, arthritis, epididymitis | + | − | Maturation arrest |
|
| |||||||||
| No Underlying Autoimmune Disease | |||||||||
|
| |||||||||
| Coppo 2004 (27) | Idiopathic | 16,M | Severe | c-ANCA | Not identified | None | + | − | Maturation arrest |
| 46,F | Mild | c-ANCA | PR3 | None | − | + | |||
| 56,F | Severe | p-ANCA | MPO,HNE | Skin-LCV | − | − | Splenomegaly | ||
| 60,M | Severe | a-ANCA | Not identified | None | + | − | Splenomegaly | ||
| 37,F | Mild | c-ANCA | Not identified | None | + | + | |||
| 44,M | Mild | c-ANCA | Not identified | None | − | − | |||
| 67,F | Severe | p-ANCA | Lactoferrin | None | − | + | Maturation arrest | ||
|
| |||||||||
| Rodrigues 2009 (29) | Cyclic Neutropenia | 38,M | Severe | c-ANCA (2) | 60kDA Protein | None | ND | ND | LGLS (1) |
| 53,F | |||||||||
Key: ND- not described; IF- immunofluorescence; ANCA- antineutrophil cytoplasmic antibodies; ANA- antinuclear antibodies; PTU- propylthiouracil, F- female; M- male; p-ANCA- perinuclear; c-ANCA- cytoplasmic; a-ANCA- atypical, MPO- myeloperoxidase; PR3- proteinase 3; HNE- human neutrophil elastase; LCV – leukocytoclastic vasculitis; GN- glomerulonephritis; SN- sensorineural; Maturation arrest - myeloid lineage on bone marrow biopsy; LGLS- large granular lymphocyte syndrome; LAC- lupus anticoagulant; ACA- anti-cardiolipin antibodies.
Degree of neutropenia: mild – 1000–1500/mm3; moderate- 500–1000/mm3; severe < 500/mm3.
Indicates biopsy-proven finding
Indicates subject level data on age and sex not provided.
Ten of 74 patients had suspected vasculitis (biopsy-proven in 6 cases). Skin lesions with leukocytoclastic vasculitis was the most commonly reported manifestation of vasculitis (8/10 suspected cases and 6/6 biopsy-proven cases) and all biopsy-proven cases were described in the setting of an underlying toxic exposure.
Antibodies other than ANCA were frequently described. Testing for antibodies directed to neutrophil surface membrane antigens can be technically challenging and is not usually performed in routine clinical practice (1). Antineutrophil membrane antibodies were detected in 8 of 16 cases in which testing occurred and were detected in at least one case in each of the three clinical categories. Antinuclear antibodies were also often present (33 of 46 cases in which such testing was reported) in all three clinical categories.
Drug-induced ANCA and neutropenia
Twenty-four cases of ANCA and neutropenia in the setting of a suspected identifiable medication or toxin were detected. Cessation of the suspected agent typically resulted in improvement in symptoms and laboratory abnormalities. Despite an extensive list of medications known to be associated with neturopenia(5), relatively few of these medications were associated with both ANCA and neutropenia.
Propylthiouracil (PTU) was a commonly implicated medication. P-ANCA with specificity for MPO was frequently reported. In a series of 56 patients with Grave's disease treated with PTU, MPO-ANCA was detected in 21 cases (38%) and neutropenia occurred in 3 of these cases (5%) (6). Akamizu et al. conducted in vitro cytotoxicity tests on serum from a patient who developed neutropenia and ANCA while being treated with PTU and demonstrated that ANCA lysed neutrophils via a complement-dependent mechanism but not by antibody-dependent cell-mediated cytotoxicity (7). Antineutrophil membrane antibodies and biopsy-proven vasculitis have been reported in cases of PTU exposure (8–10). Methimazole has also been implicated as causing development of ANCA and neutropenia (11).
Cases of lupus-like syndromes with overlapping features of systemic vasculitis have been described in association with minocycline and hydralazine. Ahmed et al. report an 18 year old patient who developed moderate neutropenia, c-ANCA with specificity to PR3, high-titer ANA, and constitutional symptoms while taking minocycline for acne (12). Sangala et al. described a patient with SLE taking hydralazine who developed biopsy-proven, pauci-immune glomerulonephritis and pancytopenia (13). The neutropenia was attributed to a lupus-like syndrome and resolved with cessation of hydralazine. Testing for antineutrophil membrane antibodies was not performed in either case.
Recently, an association with neutropenia and ANCA has been reported in users of cocaine adulterated with levamisole. Levamisole was developed as an antihelminth medication and is known to have immunostimulating effects with production of autoantibodies (14). Knowles et al. describe 60 cases of severe neutropenia associated with cocaine tainted with levamisole (15). Four of 5 cases tested for ANCA were positive (2 for c-ANCA; 2 for p-ANCA), and an additional case had detectable antineutrophil membrane antibodies. An overlap of clinical features seems to define cases of exposure to levamisole/cocaine with findings including: severe neutropenia; ANCA production with antibodies to PR3, MPO, and/or human neutrophil elastase; purpura with a predilection for the earlobes; antiphospholipid antibodies; and necrotic skin lesions with a mixed pathologic pattern of leukocytocalstic vasculitis and microthrombus (16–19). Antineutrophil membrane antibodies were present in one of two cases of ANCA and neutropenia in which testing occurred (18). Independent of cocaine exposure, a case of ANCA and neutropenia has been described in a child being treated with levamisole as adjuvant therapy for nephrotic syndrome (20).
Underlying autoimmune disease with ANCA and neutropenia
ANCA and neutropenia has been reported in association with other active autoimmune diseases including Felty's syndrome, autoimmune liver diseases, and Sjögren's syndrome. ANCA immunofluorescence patterns were usually atypical and vasculitis was infrequently described.
Felty's syndrome, a clinical triad of rheumatoid arthritis, neutropenia, and splenomegaly, has been associated with ANCA. Juby et al. report 33% prevalence of ANCA in a series of 32 patients with Felty's syndrome with severe neutropenia (21). Immunofluorescence staining showed either p-ANCA or an atypical pattern; however, testing specific ANCA antigens was not performed. Coremans et al. detected ANCA in 23 of 30 (77%) patients with Felty's syndrome (22). Specificity to lactoferrin was detected in 50% of patients with Felty's syndrome compared to 4% of a comparison group of patients with rheumatoid arthritis without Felty's syndrome. A high frequency of extra-articular disease has also been observed in Felty's syndrome with up to 28% prevalence of vasculitis reported in one series (23).
ANCA and neutropenia has also been reported in the context of other autoimmune diseases. Autoimmune liver diseases, including autoimmune hepatitis and primary sclerosis cholangitis, have been associated with non-specific ANCA, presence of antineutrophil membrane antibodies, and severe neutropenia in the absence of vasculitis (24–26). ANCA and severe neutropenia has also been described in three cases of Sjögren's syndrome, and in one case, cutaneous vasculitis and antineutrophil membrane antibodies were reported (27–28).
ANCA and chronic neutropenia without underlying autoimmune disease
Detection of ANCA has been reported in 9 cases of chronic neutropenia where no other infectious, toxic, or autoimmune etiology was identified. In a series of 9 patients, Coppo et al. described an association with ANCA and non-cyclic neutropenia in 7 patients without an identifiable underlying autoimmune disease (27). ANCA immunofluorescence patterns were mostly atypical with an unidentifiable antigen on ELISA testing, although PR3 was detected in one patient. Antineutrophil membrane antibodies were detected in 3 of 7 cases. One patient in the series developed leukocytoclastic vasculitis, and many of these patients had additional findings suggestive of a potential underlying autoimmune mechanism including hemolytic anemia, thrombocytopenia, and other circulating autoantibodies. Rodrigues et al. demonstrated ANCA in the sera of two patients with cyclic neutropenia and no other underlying autoimmune disease; these ANCA reacted with a novel 60kDa protein but not to PR3 or MPO (29).
DISCUSSION
This case and the existing literature support an association between ANCA and neutropenia. This association can occur in cases of drug exposure, in association with other active autoimmune diseases, and in cases of chronic neutropenia without underlying autoimmune disease. Reported ANCA immunoflouresence patterns were varied with approximately equal proportions of atypical and perinuclear patterns and fewer cytoplasmic patterns. Antibodies to either PR3 or MPO, the two antigens used to diagnose and categorize AAV (30), were commonly observed in cases of ANCA-associated neutropenia, yet few of these cases developed clinical evidence of vasculitis. When vasculitis did occur, it was usually limited to skin involvement in the setting of underlying exposure to a toxin. Whether a causal relationship exists between ANCA and neutropenia is unclear, and the presence of additional antibodies, especially antineutrophil membrane antibodies, complicates issues of causality.
The present case is unique because neutropenia was associated with systemic vasculitis and not only cutaneous disease, and occurred without evidence of an underlying toxic exposure. This patient was not taking any medications known to cause drug-induced vasculitis and repeated urine toxicology screening tests were negative for cocaine. The manifestations of inflammatory arthritis (31), biopsy-proven cutaneous vasculitis (32), sensorineural hearing loss responsive to immunosuppressants (33), and epididymitis (34), combined with the ANCA test results, provide compelling evidence for a diagnosis of AAV with multi-organ involvement. It is notable that this patient did not develop sinonasal, pulmonary, or renal disease, which are usual organ systems affected in the commonly definable subsets of AAV [granulomatosis with polyangiitis (Wegener's), microscopic polyangiitis, and Churg Straus syndrome].
This case and the existing literature raise interesting questions about the pathophysiology of neutropenia in association with ANCA and antineutrophil membrane antibodies. Potential underlying mechanisms for autoimmune neutropenia include cellular or humoral suppression of granulopoesis, antibody-mediated sequestration or margination of neutrophils, and peripheral destruction of neutrophils (35). Evidence supporting one or more of these hypotheses was present in the current case and many of the previously reported cases. Maturation arrest of myeloid precursors was seen on bone marrow biopsy in the present case and several other cases (12, 24–25, 27–28) suggesting possible cellular or immune-mediated central marrow suppression. Cases in which splenomegaly was found suggest the potential for neutrophil sequestration (9, 20–22, 27, 36). Evidence for peripheral destruction of neutrophils was supported by the presence of antibodies to neutrophil antigens and by the findings of hypercellularity on bone marrow biopsy (7, 13, 37).
Antineutrophil membrane antibodies, directed against neutrophil cell surface antigens, are known to cause neutropenia via complement-mediated cytotoxicity (38) and phagocytosis (39). Whether ANCA are directly causal for neutropenia is unclear. In the present case, unlike in most other cases of ANCA and neutropenia reported in the literature, both ANCA and antineutrophil membrane antibodies were measured longitudinally in periods of severe neutropenia and recovery. ANCA and antineutrophil membrane antibodies were detected in the patient's serum during periods of severe neutropenia. IgM antineutrophil membrane antibodies were detected during a period of absolute neutropenia but were no longer detectable during neutrophil recovery. In contrast, ANCA titers did not seem to correlate with neutrophil counts. These data suggest that antineutrophil membrane antibodies, rather than ANCA, were causal for neutropenia but do not exclude the possibility that the underlying etiology was multi-factorial and that ANCA may have been contributory.
The observation that the patient in current report had several weeks of recurrent fever and neutropenia before developing features of systemic vasculitis supports a hypothesis that autoimmune neutropenia may induce the formation of ANCA. Lysis of neutrophils by antineutrophil membrane antibodies and/or ANCA antigen migration to the neutrophil cell surface promoted by endogenous pyrogens (40–41), may expose the immune system to cytoplasmic antigens including PR3 and MPO (41–42) in an immunogenic environment, leading to the generation of ANCA and subsequent systemic vasculitis (43). It should be noted, however, that the pathogenicity of ANCA in AAV is somewhat controversial (44). Only a few of the patients reported in the literature with ANCA and neutropenia developed vasculitis despite developing antibodies to PR3 and MPO, suggesting that factors in addition to ANCA may be necessary in the pathogenesis of AAV. Alternatively vasculitis may be under-recognized when it occurs in association with neutropenia because the clinical features of vasculitis may be atypical in this setting, as evidenced by the present case.
Neutrophils are felt to play a primary role in the acute injury of AAV (45). In the present case, symptoms of vasculitis occurred both in periods of absolute neutropenia and in times of neutrophil recovery. The paradox of vasculitis in the setting of absolute neutropenia may be explained by the fact that circulating levels of neutrophils may not necessarily reflect neutrophil burden in other tissues. For example, the currently-described patient developed an inflammatory ankle effusion with neutrophil predominance while the absolute neutrophil count was 0.
Treating a patient with severe neutropenia with medications that can both cause neutropenia and increase the risk of infection is a major clinical challenge but was justified in this case given the suspected autoimmune etiology of the neutropenia. The patient experienced fewer neutropenic episodes following the initiation of treatment with immunosuppressive therapy. Repeated dosing of rituximab was particularly efficacious in achieving sustained disease remission compared to other immunosuppressant agents.
ANCA is associated with autoimmune neutropenia but causality has not been established. ANCA testing should be considered in cases of unexplained neutropenia, and a positive ANCA should increase clinical suspicion for possible underlying exposures to toxins or autoimmune diseases. Although clinically-apparent vasculitis appears to be uncommon in ANCA-associated neutropenia, cutaneous and systemic manifestations have been reported. Further investigation into the interaction between neutrophils and ANCA may provide important insights into the pathophysiology underlying autoimmune neutropenia and AAV.
ACKKNOWLEDGMENTS
The authors thank Janice G. McFarland, MD and her colleagues at the Blood Center of Wisconsin Platelet and Neutrophil Immunology Laboratory for performing Granulocyte Immunoflourescence Testing as well as Ivona Aksentijevich, MD and her colleagues at the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health for performing genetic testing for periodic fever syndromes.
ROLE OF THE FUNDING SOURCE P Grayson - NIH T32 (AR 007598) Training Program in Rheumatic Disease
P Monach – Arthritis Foundation Arthritis Investigator Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS None
REFERENCES
- 1.Capsoni F, Sarzi-Puttini P, Zanella A. Primary and secondary autoimmune neutropenia. Arthritis Res Ther. 2005;7(5):208–14. doi: 10.1186/ar1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niles JL. Antineutrophil cytoplasmic antibodies in the classification of vasculitis. Annu Rev Med. 1996;47:303–13. doi: 10.1146/annurev.med.47.1.303. [DOI] [PubMed] [Google Scholar]
- 3.Davoren A, Curtis BR, Shulman IA, Mohrbacher AF, Bux J, Kwiatkowska BJ, et al. TRALI due to granulocyte-agglutinating human neutrophil antigen-3a (5b) alloantibodies in donor plasma: a report of 2 fatalities. Transfusion. 2003 May;43(5):641–5. doi: 10.1046/j.1537-2995.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 4.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010 Jul 15;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt V, Saleem A. Review: Drug-induced neutropenia--pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004 Spring;34(2):131–7. [PubMed] [Google Scholar]
- 6.Sera N, Ashizawa K, Ando T, Abe Y, Ide A, Usa T, et al. Treatment with propylthiouracil is associated with appearance of antineutrophil cytoplasmic antibodies in some patients with Graves' disease. Thyroid. 2000 Jul;10(7):595–9. doi: 10.1089/thy.2000.10.595. [DOI] [PubMed] [Google Scholar]
- 7.Akamizu T, Ozaki S, Hiratani H, Uesugi H, Sobajima J, Hataya Y, et al. Drug-induced neutropenia associated with anti-neutrophil cytoplasmic antibodies (ANCA): possible involvement of complement in granulocyte cytotoxicity. Clin Exp Immunol. 2002 Jan;127(1):92–8. doi: 10.1046/j.1365-2249.2002.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada A, Sato K, Hara M, Tochimoto A, Takagi S, Hizuka N, et al. Propylthiouracil-induced lupus-like syndrome developing in a Graves' patient with a sibling with systemic lupus erythematosus. Intern Med. 2002 Dec;41(12):1204–8. doi: 10.2169/internalmedicine.41.1204. [DOI] [PubMed] [Google Scholar]
- 9.Ozkok A, Salman S, Agan M, Yavuz AS, Yarman S, Boztepe H, et al. Propylthiouracil induced anti-neutrophil cytoplasmic antibody-associated vasculitis with bone marrow plasmacytosis and granulocytopenia. Chin Med J (Engl) 2009 May 5;122(9):1112–4. [PubMed] [Google Scholar]
- 10.Farah RE, Shay MD. Symmetrical peripheral gangrene and neutropenia following propylthiouracil. Ann Pharmacother. 2006 Jun;40(6):1211. doi: 10.1345/aph.1G698. [DOI] [PubMed] [Google Scholar]
- 11.Kawachi Y, Nukaga H, Hoshino M, Iwata M, Otsuka F. ANCA-associated vasculitis and lupus-like syndrome caused by methimazole. Clin Exp Dermatol. 1995 Jul;20(4):345–7. doi: 10.1111/j.1365-2230.1995.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed F, Kelsey PR, Shariff N. Lupus syndrome with neutropenia following minocycline therapy - a case report. Int J Lab Hematol. 2008 Dec;30(6):543–5. doi: 10.1111/j.1751-553X.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 13.Sangala N, Lee RW, Horsfield C, Goldsmith DJ. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010 Jun;42(2):503–6. doi: 10.1007/s11255-009-9627-9. [DOI] [PubMed] [Google Scholar]
- 14.Rongioletti F, Ghio L, Ginevri F, Bleidl D, Rinaldi S, Edefonti A, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999 May;140(5):948–51. doi: 10.1046/j.1365-2133.1999.02833.x. [DOI] [PubMed] [Google Scholar]
- 15.Knowles L, Buxton JA, Skuridina N, Achebe I, Legatt D, Fan S, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J. 2009;6:30. doi: 10.1186/1477-7517-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh NM, Green PJ, Burlingame RW, Pasternak S, Hanly JG. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010 Aug 24; doi: 10.1111/j.1600-0560.2010.01613.x. [DOI] [PubMed] [Google Scholar]
- 17.Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med. 2010 Jun 1;152(11):758–9. doi: 10.7326/0003-4819-152-11-201006010-00026. [DOI] [PubMed] [Google Scholar]
- 18.Czuchlewski DR, Brackney M, Ewers C, Manna J, Fekrazad MH, Martinez A, et al. Clinicopathologic features of agranulocytosis in the setting of levamisole-tainted cocaine. Am J Clin Pathol. 2010 Mar;133(3):466–72. doi: 10.1309/AJCPOPQNBP5THKP1. [DOI] [PubMed] [Google Scholar]
- 19.Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009 Feb 17;150(4):287–9. doi: 10.7326/0003-4819-150-4-200902170-00102. [DOI] [PubMed] [Google Scholar]
- 20.Barbano G, Ginevri F, Ghiggeri GM, Gusmano R. Disseminated autoimmune disease during levamisole treatment of nephrotic syndrome. Pediatr Nephrol. 1999 Sep;13(7):602–3. doi: 10.1007/s004670050753. [DOI] [PubMed] [Google Scholar]
- 21.Juby A, Johnston C, Davis P, Russell AS. Antinuclear and antineutrophil cytoplasmic antibodies (ANCA) in the sera of patients with Felty's syndrome. Br J Rheumatol. 1992 Mar;31(3):185–8. doi: 10.1093/rheumatology/31.3.185. [DOI] [PubMed] [Google Scholar]
- 22.Coremans IE, Hagen EC, van der Voort EA, van der Woude FJ, Daha MR, Breedveld FC. Autoantibodies to neutrophil cytoplasmic enzymes in Felty's syndrome. Clin Exp Rheumatol. 1993 May–Jun;11(3):255–62. [PubMed] [Google Scholar]
- 23.Campion G, Maddison PJ, Goulding N, James I, Ahern MJ, Watt I, et al. The Felty syndrome: a case-matched study of clinical manifestations and outcome, serologic features, and immunogenetic associations. Medicine (Baltimore) 1990 Mar;69(2):69–80. [PubMed] [Google Scholar]
- 24.Wehbe AM, Johannsson B, Raife TJ, Bleile M, Bell A, Curtis BR, et al. Severe autoimmune neutropenia associated with acute autoimmune hepatitis. Int J Hematol. 2010 May;91(4):673–8. doi: 10.1007/s12185-010-0557-1. [DOI] [PubMed] [Google Scholar]
- 25.Cuadrado A, Aresti S, Cortes MA, Gomez-Ortega JM, Salcines JR. Autoimmune hepatitis and agranulocytosis. Dig Liver Dis. 2009 Jul;41(7):e14–6. doi: 10.1016/j.dld.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Hanawa NTA, Fukami M, Miura R, Goto H, Tashiro H, et al. Autoimmune neutropenia due to antineutrophil antibodies in a patients with primary sclerosing cholangitis. Clin J Gastroenterol. 2010;3:149–54. doi: 10.1007/s12328-010-0145-1. [DOI] [PubMed] [Google Scholar]
- 27.Coppo P, Ghez D, Fuentes V, Bengoufa D, Oksenhendler E, Tribout B, et al. Antineutrophil cytoplasmic antibody-associated neutropenia. Eur J Intern Med. 2004 Nov;15(7):451–9. doi: 10.1016/j.ejim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan K, Ross CW, Bockenstedt PL, Adams PT. Successful treatment of autoimmune neutropenia with recombinant human granulocyte-colony stimulating factor (R-metHuGCSF) Clin Lab Haematol. 1997 Jun;19(2):105–9. doi: 10.1046/j.1365-2257.1997.d01-275.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues CE, Velloso ER, Pereira RM, Bonfa E, Teixeira FK, Bueno C, et al. A novel 60kDa reactivity in cyclic neutropenia: High titer cytoplasmic ANCA immunostaining pattern and negative anti-proteinase-3 antibody. Joint Bone Spine. 2010 Nov 18; doi: 10.1016/j.jbspin.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Hagen EC, Daha MR, Hermans J, Andrassy K, Csernok E, Gaskin G, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int. 1998 Mar;53(3):743–53. doi: 10.1046/j.1523-1755.1998.00807.x. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992 Mar 15;116(6):488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 32.Barksdale SK, Hallahan CW, Kerr GS, Fauci AS, Stern JB, Travis WD. Cutaneous pathology in Wegener's granulomatosis. A clinicopathologic study of 75 biopsies in 46 patients. Am J Surg Pathol. 1995 Feb;19(2):161–72. [PubMed] [Google Scholar]
- 33.Bakthavachalam S, Driver MS, Cox C, Spiegel JH, Grundfast KM, Merkel PA. Hearing loss in Wegener's granulomatosis. Otol Neurotol. 2004 Sep;25(5):833–7. doi: 10.1097/00129492-200409000-00030. [DOI] [PubMed] [Google Scholar]
- 34.Lee SS, Tang SH, Sun GH, Yu CP, Jin JS, Chang SY. Limited Wegener's granulomatosis of the epididymis and testis. Asian J Androl. 2006 Nov;8(6):737–9. doi: 10.1111/j.1745-7262.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 35.Balint GP, Balint PV. Felty's syndrome. Best Pract Res Clin Rheumatol. 2004 Oct;18(5):631–45. doi: 10.1016/j.berh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Jani AL, Hamilos D. Bloody diarrhea, fever, and pancytopenia in a patient with active ulcerative colitis. Ann Allergy Asthma Immunol. 2003 Apr;90(4):383–8. doi: 10.1016/S1081-1206(10)61820-0. [DOI] [PubMed] [Google Scholar]
- 37.Finucane FM, O'Connell J, Kinsley BT. Propylthiouracil induced C-ANCA positive agranulocytosis complicating Graves' thyrotoxicosis in pregnancy. Ir J Med Sci. 2008 Mar;177(1):69–71. doi: 10.1007/s11845-007-0055-5. [DOI] [PubMed] [Google Scholar]
- 38.Rustagi PK, Currie MS, Logue GL. Activation of human complement by immunoglobulin G antigranulocyte antibody. J Clin Invest. 1982 Dec;70(6):1137–47. doi: 10.1172/JCI110712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadley AG, Holburn AM, Bunch C, Chapel H. Anti-granulocyte opsonic activity and autoimmune neutropenia. Br J Haematol. 1986 Jul;63(3):581–9. doi: 10.1111/j.1365-2141.1986.tb07536.x. [DOI] [PubMed] [Google Scholar]
- 40.Muller Kobold AC, Kallenberg CG, Tervaert JW. Leucocyte membrane expression of proteinase 3 correlates with disease activity in patients with Wegener's granulomatosis. Br J Rheumatol. 1998 Aug;37(8):901–7. doi: 10.1093/rheumatology/37.8.901. [DOI] [PubMed] [Google Scholar]
- 41.Csernok E, Ernst M, Schmitt W, Bainton DF, Gross WL. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994 Feb;95(2):244–50. doi: 10.1111/j.1365-2249.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilligan HM, Bredy B, Brady HR, Hebert MJ, Slayter HS, Xu Y, et al. Antineutrophil cytoplasmic autoantibodies interact with primary granule constituents on the surface of apoptotic neutrophils in the absence of neutrophil priming. J Exp Med. 1996 Dec 1;184(6):2231–41. doi: 10.1084/jem.184.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002 Oct;110(7):955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falk RJ, Hoffman GS. Controversies in small vessel vasculitis--comparing the rheumatology and nephrology views. Curr Opin Rheumatol. 2007 Jan;19(1):1–9. doi: 10.1097/BOR.0b013e328011cb80. [DOI] [PubMed] [Google Scholar]
- 45.Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005 Jul;167(1):39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]