Table 3.
PBu3-Catalyzed [3 + 2] Cycloadditions of Azomethine Imines 1 with the Allenoate 2aa
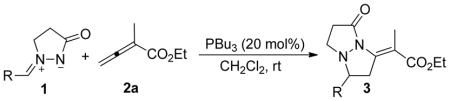 | ||||
|---|---|---|---|---|
| entry | R | time (h) | product | yield (%)b |
| 1 | Ph (1b) | 24 | 3ba | 97 |
| 2 | 4-MeC6H4 (1c) | 24 | 3ca | 85 |
| 3 | 4-i-PrC6H4 (1d) | 24 | 3da | 89 |
| 4 | 4-OMeC6H4 (1e) | 30 | 3ea | 77 |
| 5 | 4-FC6H4 (1f) | 24 | 3fa | 91 |
| 6 | 4-ClC6H4 (1g) | 20 | 3ga | 93 |
| 7 | 4-BrC6H4 (1h) | 20 | 3ha | 97 |
| 8 | 4-CNC6H4 (1i) | 12 | 3ia | 87 |
| 9 | 4-CF3C6H4 (1j) | 24 | 3ja | 98 |
| 10 | 3-NO2C6H4 (1k) | 24 | 3ka | 97 |
| 11 | 2-NO2C6H4 (1l) | 24 | 3la | 94 |
| 12c | 2-MeC6H4 (1m) | 48 | 3ma | 70 |
| 13c | 2-PhC6H4 (1n) | 48 | 3na | 50 |
| 14c | 1-naphthyl (1o) | 48 | 3oa | 57 |
| 15 | 2-naphthyl (1p) | 24 | 3pa | 96 |
| 16 | 4-pyridyl (1q) | 20 | 3qa | 86 |
| 17 | 2-furanyl (1r) | 20 | 3ra | 99 |
| 18 | cyclohexyl (1s) | 36 | 3sa | 55 |
| 19 | n-Bu (1t) | 72 | 3ta | 41 |
| 20 | i-Pr (1u) | 72 | 3ua | 48 |
1.2 equiv of allenoate was used.
Isolated yield.
The reaction was run at 40 °C.
