Figure 4.
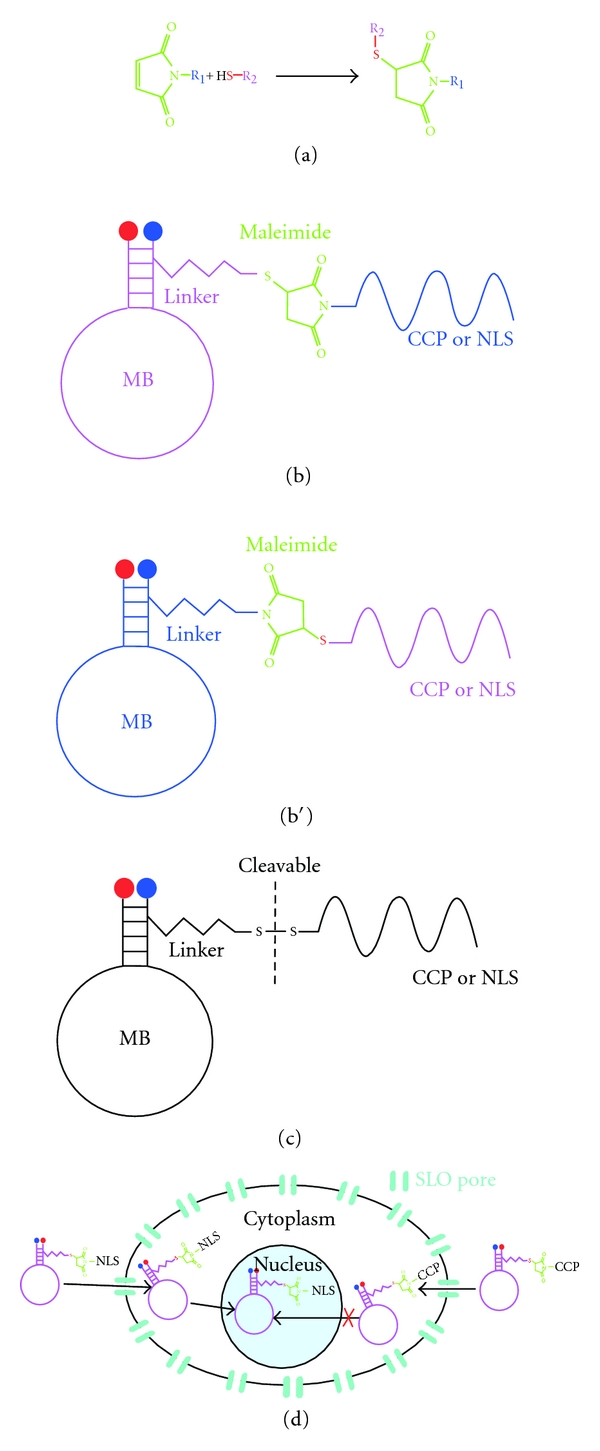
MB linked to penetrating peptides. (a) Reaction of conjugation among the maleimide molecule with a carrier group (R1) and the thiol group of another molecule. This reaction may possibly link MB-peptide and occurs at a pH between 6.5–7.5. (b) One possibility using the maleimide-thiol system is that the peptide (NLS or CCP) it is linked to the maleimide across the secondary amine and reacts with a sulfhydryl group at the terminus of the MB hydrocarbon linker. (b′) The other possibility for the maleimide-thiol system is that the maleimide is linked at the terminus of the MB hydrocarbon linker and reacts with a thiol group at the peptide (NLS or CCP). (c) The link between the hydrocarbon linker of the MB and the peptide also could be across a disulphide bridge, in this case exists the possibility to cleave the bond in a reducing environment. (d) Using SLO to make permeable the cytoplasmic membrane is possible to introduce a MB linked to an NLS peptide to the cytoplasm with the objective that it be transported into the nucleus by the cellular machinery. For a CCP-linked MB the entrance to the nucleus is impossible.
