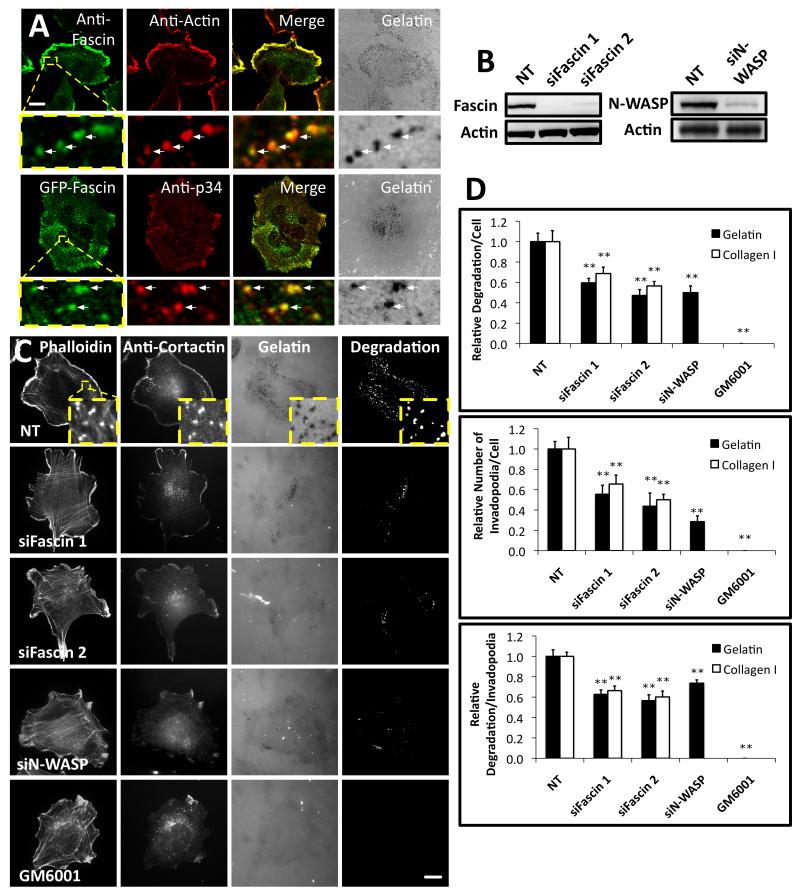
Figure 1. Fascin is important for invadopodia assembly and matrix degradation.
(A) Top, CHL-1 human melanoma cells on Oregon Green® 488 gelatin matrix (grey) with anti-fascin (primary 55K2) and anti-actin. Bottom, cells expressing GFP-fascin on Alexa594 gelatin matrix (grey) with anti-p34-Arc (ARPC2). Arrows- invadopodia. Bar, 10μm. (B) Western blots of lysates from CHL-1 cells with anti-fascin (Left), anti-N-WASP (Right) or anti-actin (Loading control, left and right) (C) CHL-1 cells expressing siRNAs as indicated or treated with 5 μM GM6001 on Oregon Green® 488 gelatin matrix with rhodamine phalloidin (F-actin) and anti-cortactin. Bars, 10μm. (D) Top: Relative area of degradation per cell on gelatin (Black) and Collagen I (White). Middle: number of invadopodia per cell on gelatin (Black) and Collagen I (White). Bottom: area of degradation per invadopodium of the ten biggest degradations per cell on gelatin (Black) and Collagen I (White). All error bars indicate (Mean ± SEM). **, P< 0.01 by t-test. Invadopodia were defined as puncta enriched for actin, cortactin and gelatin degradation. Similar experiments done with A375MM or MDA-MB231 cells are shown in Figure S1. Detailed analysis of fascin localization at invadopodia is shown in Figure S2. Movie 1 shows dynamics of actin comets at invadopodia and 3D reconstruction of fascin localization in comets.