Figure 2.
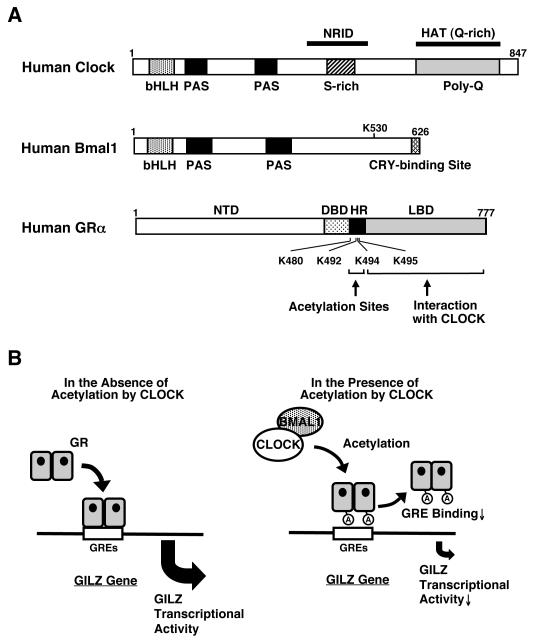
Linearized structures of the human Clock, Bmal1 and GR molecules and the regulation GR transcriptional activity by Clock .
(A) Human Clock and Bmal1 are 847 and 626 amino acid proteins, respectively, that contain one basic helix-loop-helix (bHLH) and two Per-Amt-Sim (PAS) domains in their N-terminal portion. Clock has also one nuclear receptor interacting domain (NRID) in its middle portion and a histone acetyltransferase domain (HAT) in its C-terminal area, while Bmal1 has a CRY-binding site in its C-terminal end. A serine-rich (S-rich) and a glutamine-rich (Q-rich) region, the latter containing a poly-glutamine (polyQ) stretch, are also shown. The GR has 3 main functional domains, the amino terminal, DNA-binding and ligand-binding domains and a lysine cluster in its hinge region.
(B) Clock physically interacts with the ligand-binding domain of the GR through the domain enclosed in its C-terminal part and suppresses GR-induced transcriptional activity by acetylating via its intrinsic HAT activity a lysine cluster located in the GR hinge region. Acetylation by Clock reduces the affinity of the GR to its cognate DNA sequences, the GREs.
A: acetylation, Bmal1: brain-muscle-arnt-like protein 1, DBD: DNA-binding domain, GR: glucocorticoid receptor, GRE: glucocorticoid response element, HR: hinge region, K: lysine residue, LBD: ligand-binding domain, NTD: N-terminal domain C: A heuristic model of the physiologic implications of this study.