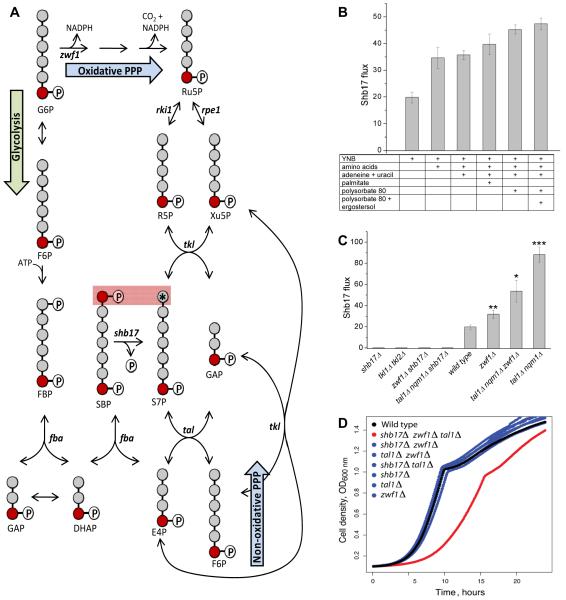
Figure 5.
Shb17 feeds carbon into the non-oxidative pentose phosphate pathway.
(A) Flux through Shb17 into S7P can be measured using [6-13C1]-glucose. [6-13C1]-glucose leads to [7-13C1]-S7P when S7P is made via the oxidative PPP or the non-oxidative PPP. However, when S7P is produced from SBP via Shb17, a fraction of the S7P pool is doubly labeled: [1,7-13C2]-S7P. Flux is calculated based on the measured isotopic distribution of SBP and S7P.
(B) Flux through Shb17 is increased by supplementation with nutrients whose endogenous production requires NADPH, and thus drives oxidative PPP flux. All measurements are performed in wild type yeast. YNB is yeast nitrogen base without amino acids plus 2% glucose. Supplementation with amino acids includes 17 amino acids. Data shown is the arithmetic mean ± SE of N=3 technical replicates.
(C) Effects of PPP gene deletions on Shb17 flux. Deletions are: glucose 6-phosphate dehydrogenase zwf1Δ; transketolase tkl1Δ/ tkl2Δ; transaldolase is tal1Δ/nqm1Δ. Less than 1% doubly labeled S7P was observed in any shb17Δ strain in all measured conditions. All strains were grown in YNB + 2% glucose and supplements as required: methionine for zwf1Δ; synthetic complete media including aromatic amino acids for tkl1Δ/tkl2Δ.
(C) Triple deletion of the sedoheptulose bisphosphatase SHB17, the glucose-6-phosphate dehydrogenase ZWF1, and the transaldolase TAL1, causes a growth defect. Optical density was measured during growth at 30 degrees C in YPD. Growth data are presented in Supplemental Table 3.