Abstract
Purpose: Little is known about limitations in physical function across BMI categories in middle aged women using both self-report and performance-based measures. Furthermore, the impact of BMI on the measurement of function has not been explored. The purpose of this study was to assess physical function in adult women across BMI categories using self-report and performance-based measures and determine the influence of BMI on the relationship between the measures. Methods: Fifty sedentary females (10 in each BMI category: normal weight, overweight, obese class I, II, and III) aged 51.2 ± 5.4 years participated. Assessments included demographics, past medical history, physical activity level, BMI, and self-report (Late Life Function and Disability Instrument) and performance-based measures of physical function (6-Minute Walk Test, timed chair rise, gait speed). Physical function was compared between BMI categories using analysis of variance. The influence of BMI on the relationship of self-report and performance-based measures was analyzed using linear regression. Results: Compared to those that were normal weight or overweight, individuals with obesity scored lower on the self-report measure of physical function (LLFDI) for capability in participating in life tasks and ability to perform discrete functional activities. On the performance-based measures, the individuals with obesity had slower gait speed compared to the normal and overweight weight groups. For the 6-Minute Walk Test and timed chair stands, individuals with obesity had poorer performance compared to those who were normal weight. Linear regression analyses revealed that BMI attenuated the relationship between the self-report and performance-based measures by approximately 50%. Conclusions: While those with severe obesity were most impaired, adult women with less severe obesity also demonstrated significant decrements in physical function.
Key Words: obesity, physical function
INTRODUCTION
Obesity is a major public health problem in the United States with estimates of over 65% of adults being overweight, defined as a body mass index (BMI) greater than or equal to 25.0 kg/m2. Of these individuals, over 30% are considered obese (BMI ≥ 30 kg/m2).1 This is concerning because the health and economic burdens of obesity are vast. Numerous chronic diseases are strongly associated with obesity including hypertension, type 2 diabetes, osteoarthritis, and certain forms of cancer.2,3 In addition, obesity is an independent risk factor for cardiovascular disease and those with excess weight have higher risk for cardiac complications such as coronary heart disease, heart failure, and sudden death.4,5 In 2000, the total cost of obesity for the United States was estimated to be $117 billion.6 For these reasons, it is imperative that all health care professionals, including physical therapists, are able to effectively evaluate and treat conditions related to overweight and obesity.
Among US adults, middle-aged women have the highest prevalence of obesity. It is reported that 38.2% of women aged 40 to 59 years were obese in 2007–2008 based on data from the National Health and Nutrition Examination Survey (NHANES).1 The substantial presence of obesity in the middle-aged is concerning given the association between obesity and future disability. Studies have demonstrated that obesity in young and middle age is associated with self-reported disability and poorer physical function later in life.7,8 Analysis of recent trends has shown that obesity-related disability is on the rise,9 reinforcing the need for a better understanding of the impact of obesity on physical function in this group.
The BMI is the most common method to quantify weight across a range of body sizes in adults.10 Body mass index has been shown to be highly correlated with body fat in adult women in the NHANES surveys.11 Body mass index is calculated by dividing an individual's weight in kilograms by the height in meters squared (kg/m2). Health care professionals may use BMI to classify individuals as underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25 29.9 kg/m2), class I obese (30-34.9 kg/m2), class II obese (35 39.9 kg/m2), or class III obese (≥ 40 kg/m2). These categories of BMI were developed by the World Health Organization based on associated health risks.12
While BMI is an important clinical tool that may be used in the initial assessment of overweight and obesity,4 BMI does not distinguish between fat and lean mass and thus, has several limitations. Body mass index may overestimate body fat in those with larger muscle mass, such as athletes, and may underestimate body fat in those that have lost muscle mass.13 Furthermore, studies suggest there is a need for population-specific BMI classifications because adiposity associated with a given level of BMI varies by race, age, and gender.14,15
Weight reduction is recommended for adults with obesity and exercise has been shown to be an important component of weight loss and weight maintenance programs.16 Various studies have shown the benefits of weight reduction including an improvement in cardiovascular risk factors such as a decrease in blood pressure,17 an improvement in lipid profile and glucose tolerance,18,19 and reduced inflammation.20 Furthermore, there is evidence that weight loss achieved through diet and exercise improves physical function and health-related quality of life.21–25 In view of the widespread prevalence of obesity, its impact on physical function, and the critical role of exercise, physical therapists are well-positioned to have a substantial impact on this significant public health problem.
The Guide to Physical Therapist Practice supports the inclusion of health promotion in patient/client management including the use of screening tools, such as BMI, in the physical therapist examination.26 Furthermore, APTA's vision statement (Vision 2020) supports the role of physical therapists in health promotion advocating for the physical therapist to be the practitioner of choice “for the diagnosis of, interventions for, and prevention of impairments, functional limitations, and disabilities related to movement, function, and health.”27 Physical therapists can apply health promotion practice through the determination of a patient or client's body size using anthropometric measures, such as BMI. This knowledge could then be used to identify the individual's associated health risk status and may consequently result in an intervention, such as physical activity counseling, to reduce disease risk.
Despite evidence relating obesity to impaired physical function, there are several limitations in the current body of research. The majority of studies investigating the relationship between BMI and physical function have focused on older adults or those with class III obesity (BMI ≥ 40 kg/m2).28–33 Thus, little is known about the impact of BMI on physical function in middle-aged adults across the broader continuum of weight ranges. In addition, most studies have relied on self-report measures to assess physical function.30,31,34,35 Self-report measures may be subject to personal bias and traditional measures were not designed to capture deficits across a spectrum of functional abilities because they typically only assess whether an individual is able to perform a task or not.36 Finally, studies reveal a gender discrepancy in the impact of obesity on physical function with women more negatively affected by excess body weight compared to men.37–40 Given the increased prevalence of disability in middle-aged adults and the disproportionate negative effect of obesity on women, there is a need to more precisely define the impact of BMI on physical function in this group.41 This information would assist in determining the functional consequences of obesity in middle-aged women.
Previous investigations comparing self-report and performance-based measures of function have shown only moderate correlations suggesting that each assesses a different construct of physical function.36 Specifically, self-report is an assessment of what an individual perceives he/she can do and the performance-based measure is an assessment of actual ability.36 There is the suggestion in previous research that compared to those that accurately report their function, women who under-report ability are more likely to be overweight.42 However, the association between perception of function and ability in those with excess weight has not been explored. The purpose of this study was two-fold: (1) to assess physical function in adult women across the range of BMI categories using self-report and performance-based measures, and (2) to examine the impact of BMI on the relation of self-report and performance-based measures of physical function.
METHODS AND PROCEDURES
Study population
To successfully obtain subjects for this study, the following recruitment techniques were utilized: advertisement in local papers; Internet sites; and newsletters, mailings, and posting advertisements in targeted areas. Subjects were included in this cross-sectional study if they were female, 40-60 years old, sedentary (defined as reporting exercising less than 3 days/week for less than 20 minutes/day over the previous 6 months), and had the ability to walk without an assistive device (n=50). To ensure representation across the span of BMI levels, 10 normal weight (BMI between 18.5 and 24.9 kg/m2), 10 overweight (BMI between 25.0 to 29.9 kg/m2), 10 class I obese (BMI between 30.0 to 34.9 kg/m2), 10 class II obese (BMI between 35.0-39.9 kg/m2), and 10 class III obese (BMI ≥ 40 kg/m2) 43 individuals were recruited. The sample was restricted to this age range and gender to control for potential confounding variables that may impact physical function. Subjects were excluded if they had any of the following conditions: neuromuscular disorders that impaired movement, cancer with active treatment, hospitalization for a life-threatening illness or major surgery in the past 6 months, chest pain with activity, or a cardiac event such as a heart attack in the past 6 months. All subjects provided written informed consent and this study was approved by the Institutional Review Board at the University of Pittsburgh.
Anthropometric data were collected first, followed by self-report and interview administered questionnaires. Performance-based measures were administered after the self-report measures to minimize the influence of the persons' performance on their self-reporting of function. Data were collected by a licensed physical therapist in Forbes Tower on the University of Pittsburgh campus and in Birmingham Towers at the University of Pittsburgh Physical Activity and Weight Management Research Center.
Weight and body mass index
Body weight was determined using a Tanita Model TBF-310 GS Weight Scale (Tanita Corporation of America, Inc., Arlington Heights, IL). Subjects wore lightweight clothing and were weighed without shoes. Height was determined through the use of a wall-mounted stadiometer after shoes were removed. The subject's BMI was computed from the height and weight measurements (kg/m2).
Body composition
Measurement of body composition to determine percentage body fat using bioelectrical impedance analysis (BIA) was performed with a Tanita Model TBF-310 GS Weight and Body Composition Scale. Bioelectrical impedance analysis was assessed using standards recommended by the manufacturer.
Self-report measure
Late Life Function and Disability Instrument (LLFDI): The LLFDI was used as a comprehensive assessment of two disablement constructs: functional limitations (altered ability to perform specific actions encountered in daily routines) and disability (altered performance of major life tasks and social roles).44 The disability component of the LLFDI is comprised of a frequency (performance) and limitation (capability) total score for 16 major life tasks such as preparing meals and taking care of local errands. The function component evaluates self-reported difficulty in performing 32 physical tasks such as going up and down a flight of stairs, putting on and taking off a jacket, and walking a mile. Raw scores for each component were transformed into a scaled score ranging from 0-100 using the LLFDI Scoring Software. Higher scores were representative of a higher level of function and lower level of disability. Though originally developed as a tool for assessing function and disability in older adults, the LLFDI has been validated for use in middle aged adults45 and was selected as a self-report measure of physical function because of its broad content and sensitivity in detecting deficits in function across the spectrum of disability with minimum ceiling and floor effects.44 The LLFDI has been shown to have concurrent validity with the physical functioning subscale of the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36) in older adults46 as well as concurrent and predictive validity based on moderate associations with performance-based measures of physical function such as the 400-meter walk and the Short Physical Performance Battery in adults.44
Performance-based measures
Gait Speed: Gait speed has been shown to be a quick and inexpensive clinical tool for predicting a number of health related outcomes in older adults including nursing home admission, falls, disability, and death.47 The intraclass correlation coefficient for test-retest reliability in adults and older adults has been shown to be > .90.48,49 Gait speed was determined by recording the time for each subject to walk the central 4 meters of an 8-meter course at usual, self-selected pace using a stopwatch. Gait speed was calculated as the distance (4 meter) divided by the time it took to complete the 4-meter walk in seconds. The initial and final 2 meters were excluded from the calculation to eliminate the effects of acceleration and deceleration. The gait speed was reported in meters per second (m/s). This test was repeated twice for each subject and the average of two trials was used.
Timed Chair Rise: Chair stands have been used as a performance-based measure of lower body function and have been shown to have good reliability in a sample of adults aged 35-71 years (ICC = > .80).50 Participants were seated in a rigid chair, folded their arms across their chest, and stood up straight and sat down as quickly as possible 5 times. The time to complete 5 repeated stands from the chair was recorded.
6Minute Walk Test: The 6-Minute Walk Test was administered using a standardized protocol.51 This test has been used as a measure of aerobic endurance and functional mobility in adults with and without disease and has shown to be a reliable measure with an intraclass correlation coefficient of > .90.52 Subjects walked as far as possible in 6 minutes around a series of traffic cones placed on a level corridor measuring 30.5 meters in length, taking rest periods as needed. The number of laps completed was counted by the tester. After 6 minutes, the subject was instructed to stop walking. A marker was placed on the ground and the distance walked during the last lap was measured with a tape measure. The total distance walked was recorded. Heart rate and blood pressure were recorded before and after the walk.
Measurements to describe sample
Demographics: A questionnaire that included questions about age, race or ethnicity, level of education, and past medical history was used.
Duke CoMorbidities Index: The Co-Morbidities Index is a self-report of physician-diagnosed conditions and self-reported symptoms.53
Physical Activity Level: The Paffenbarger Physical Activity Questionnaire was used to assess physical activity level of the subjects.54 This questionnaire has been validated as a tool to assess the weekly physical activity patterns. The 3 major components of the Paffenbarger Questionnaire are stairs climbed, walking, and sports and recreation. An estimate of the weekly energy expended through leisure time physical activity was calculated using the scoring system devised for this questionnaire, as described by Paffenbarger et al.54
Data Analysis
Data were screened through the examination of normality plots and outliers and by conducting tests of normality (Shapiro-Wilk). If data were skewed, nonparametric tests were used for the analysis. For continuous data, descriptive statistics are presented as means and standard deviations, and categorical data are presented as frequencies (percentages). To examine the difference in self-report and performance-based measures of physical function between BMI categories, the sample was divided based on weight category into 5 groups (normal, overweight, obese class I, II, and III). Physical function was compared between groups using analysis of variance (ANOVA). Tukey's tests served as post hoc analyses to determine significant differences as identified by ANOVA. To examine the effect of BMI on the relationship between self-report and performance-based measures of physical function, linear regression was used. To determine the impact of collinearity among the variables in the regression model, the variance inflation factor (VIF) was examined. Results of this testing revealed a VIF tolerance of 2.1 indicating the model was not affected by collinearity. All data were analyzed using statistical software SPSS version 17.0 (SPSS, Inc., Chicago, IL).
RESULTS
Table 1 provides a summary of demographic variables, physical activity level, and prevalent chronic conditions for all subjects and stratified by weight category. The mean age of the participants was 51.2 ± 5.4 years and most participants classified their race as white (66%). The majority of the subjects reported some degree of college education (90%). Several characteristics of the subjects were associated with higher BMI. Significant differences were found between weight categories for total number of co-morbid conditions (p = .02). Comparisons revealed that individuals who were classified as class I obese reported a higher total number of co-morbid health conditions compared to those that were normal weight (p = .04). There was a significant difference in prevalence of diabetes between the weight groups (p = .02) with individuals with class III obesity reporting the highest percentage compared to the other weight groups. Analysis of physical activity data revealed significant differences between weight groups in physical activity with the lowest levels found in the higher BMI categories (p = .025).
Table 1.
Demographic Characteristics for Total Subjects and for BMI Category
| Total (n=50) | Normal Weight (n=10) | Overweight (n=10) | Obese, Class I (n=10) | Obese, Class II (n=10) | Obese, Class III (n=10) | P | |
|---|---|---|---|---|---|---|---|
| (mean+SD*) | (mean+SD) | (mean+SD) | (mean+SD) | (mean+SD) | (mean+SD) | ||
| Age (y) | 51.2 ± 5.4 | 50 ± 1.8 | 50.4 ± 1.4 | 53.4 ± 1.6 | 53.6 ± 1.6 | 48.6 ± 1.7 | .16 |
| BMI (kg/m2) | 33.05 ± 7.7 | 22.7 ± .5 | 28.5 ± .3 | 32.21 ± .4 | 37.82 ± .3 | 44.03 ± 1.3 | <.001 |
| % Body Fat | 42% | 29% | 41% | 44% | 48% | 49% | <.001 |
| Ethnicity | % (n) | % group | % group | % group | % group | % group | |
| White | 66% | 70% | 60% | 80% | 50% | 70% | .67 |
| Black | 34% | 30% | 40% | 20% | 50% | 30% | |
| % | % group | % group | % group | % group | % group | ||
| College Educated | 90% | 100% | 70% | 90% | 90% | 100% | .09 |
| Physical Activity Level (kcals/week) | 639 ± 407 | 983 ± 162 | 719 ± 133 | 539 ± 101 | 480 ± 76 | 471 ± 97 | .021 |
| # Co-morbidities | 2 ± 1.7 | .5 ± .2 | 1 ±.4 | 3 ±.8 | 2 ±.5 | 2 ± .5 | .022 |
| Conditions | % | % group | % group | % group | % group | % group | |
| Angina | 2% | 0% | 0% | 10% | 0% | 0% | .40 |
| Broken Bone | 36% | 10% | 30% | 50% | 40% | 50% | .30 |
| Heart Failure | 2% | 0% | 0% | 10% | 0% | 0% | .40 |
| Depression | 36% | 20% | 30% | 60% | 50% | 30% | .22 |
| Lung Disorders | 18% | 10% | 10% | 20% | 30% | 20% | .76 |
| Arthritis | 30% | 10% | 10% | 40% | 50% | 40% | .16 |
| Osteoporosis | 4% | 0% | 0% | 10% | 0% | 10% | .54 |
| Diabetes | 12% | 0% | 20% | 0% | 0% | 40% | .02 |
| Sleep Problems | 18% | 0% | 10% | 20% | 40% | 20% | .20 |
| Cancer | 2% | 0% | 0% | 0% | 0% | 10% | .40 |
| Chronic Pain | 2% | 0% | 0% | 10% | 0% | 0% | .40 |
| Cataracts | 8% | 0% | 0% | 20% | 10% | 10% | .43 |
Standard deviation
p-value for Kruskal-Wallis test
Significant difference between normal weight and class I obese (p = .04)
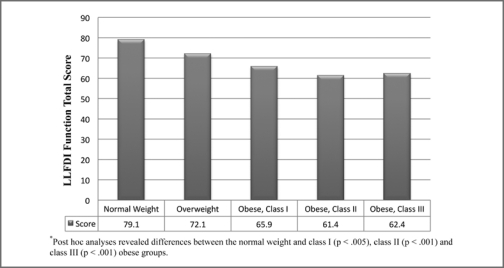
Table 2 provides a description of the self-report and performance-based measures of physical function stratified by weight group. Significant differences were found between weight categories for the capability in participating in life tasks (LLFDI disability limitation total, p < .001). Individuals with class II obesity reported decreased capability in participating in life tasks than the normal weight and overweight group. Furthermore, the individuals with class I obesity reported less capability in participating in life tasks than those who were overweight. There were no significant differences between weight groups for frequency in participating in life tasks (LLFDI disability frequency total). In the examination of ability to perform discrete functional activities, significant differences were found between weight groups (LLFDI function total, p < .001). Individuals with class I, II, and III obesity reported more difficulty in their ability to perform functional activities compared to those who were of normal weight. Figure 1 illustrates the differences between BMI categories for the LLFDI function total scores.
Table 2.
Comparison of Self-report and Performance-based Measures between BMI Categories
| MEASURE | Normal Weight | Overweight | Obese, Class I | Obese, Class II | Obese, Class III | p |
|---|---|---|---|---|---|---|
| SELF-REPORT MEASURE (LLFDI) | mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | |
| Disability Frequency | 54.20 ± 6.19 | 52.29 ± 5.27 | 52.07 ± 6.53 | 53.12 ± 5.24 | 54.09 ± 5.15 | .88 |
| Disability Limitation | 80.48 ± 13.09 | 82.43 ± 11.8 | 66.30 ± 7.98 | 64.55 ± 7.94 | 68.80 ± 10.90 | <.0011 |
| Function | 79.08 ± 8.55 | 72.10 ± 8.52 | 65.90 ± 8.13 | 61.44 ± 7.19 | 62.40 ± 6.20 | <.0012 |
| PERFORMANCE-BASED MEASURES | ||||||
| Gait Speed (m/sec) | 1.35 ± .06 | 1.20 ± .59 | 1.17 ± .07 | 1.04 ± .04 | .97 ± .04 | <.0013 |
| 6-Minute Walk (m) | 546 ± 25 | 487 ± 19 | 449 ± 28 | 450 ± 23 | 406 ± 16 | .0014 |
| Chair Stand (sec) | 10.56 ± .43 | 12.48 ± 1.15 | 13.11 ± .75 | 13.83 ± .93 | 15.64 ± 1.90 | .0155 |
Significant differences between normal weight and obese class II (p=.013), overweight and class I obese (p=.011), and overweight and class II obese weight categories (p=.004)
Significant differences between the normal weight and the obese class I (p=.004), II (p<.001) and III (p<.001) weight categories.
Significant differences between normal weight and class II obese (p = .002), normal weight and class III obese (p = .000), overweight and class III obese (p = .034)
Significant differences between normal weight and class I obese (p = .034), normal weight and class II obese (p = .038), normal weight and class III obese (p = .003)
Significant differences between normal weight and class II obese (p = .004) and normal weight and class III obese (p = .001)
Figure 1.
Differences in LLFDI function total score between BMI categories.
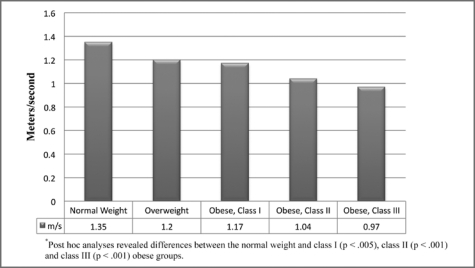
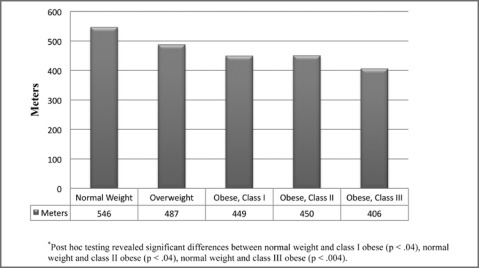
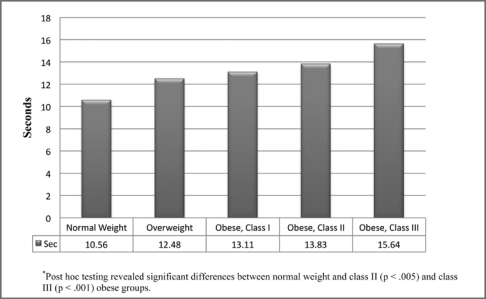
For the performance-based measures, significant differences were found between weight groups for gait speed (p < .001). Individuals with class II and III obesity had slower gait speed than those of normal weight. Those with class III obesity had slower gait speed than individuals who were overweight (Figure 2). Figure 3 illustrates the differences between weight groups for the 6-Minute Walk Test (p < .001). Individuals classified as normal weight performed better than those with class I, II, and III obesity. For the timed chair stands, significant differences were found between weight groups (p < .05). Individuals classified as normal weight had better performance than individuals with class II and III obesity (Figure 4).
Figure 2.
Gait speed stratified by weight group.
Figure 3.
6-Minute Walk Test distance stratified by weight group.
Figure 4.
Timed chair rise stratified by weight group.
Table 3 provides results of the linear regression analyses examining the effect of BMI on the relationship between self-report and performance-based measures of physical function. In the first model, the self-report measure (LLFDI advanced lower extremity score) was used as the independent variable and the performance-based measure (gait speed) as the dependent variable. The self-report measure was significantly related to performance (p < .001). In the second model, the self-report measure (LLFDI advanced lower extremity score) and BMI were added to the model as independent variables with the performance-based measure (gait speed) as the dependent variable. In the second model, BMI attenuated the relationship between the self-report measure and the performance-based measure (Beta decreased from .621 to .367, p < .03).
Table 3.
Linear Regression Analyses for Effect of BMI on Relation of Self-report and Performance-based Measures
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Beta coefficient | p-value | Beta coefficient | p-value | |
| Dependent Variable – Gait Speed | ||||
| Self-Report Measure of Physical Function | .621 | <.001 | .367 | .026 |
| Body Mass Index | — | — | −.348 | .034 |
Model 1: adjusted R2= .373, F= 30.17, p < .001, Gait Speed = .01 (LLFDI) + .53
Model 2: adjusted R2 = .419, F = 18.66, p< .001, Gait Speed = .006 (LLFDI) – .01 (BMI) + 1.1
DISCUSSION
Our results showed that participants categorized as obese felt more limited in performing life tasks compared to those that were normal weight and overweight. In addition, participants that were obese reported more difficulty performing functional activities compared to those of normal weight. Mean scaled summary scores of the self-report measure (LLFDI) have been classified into 4 different subgroups for clinical interpretation: severe limitations, moderate limitations, slight limitations, and no limitations.55 Participants that were classified as normal weight and overweight exhibited scores consistent with no limitations in capability of participating in life tasks and no limitations in performing discrete functional activities. In contrast, participants in the obese groups exhibited scores consistent with slight to moderate limitation in each of these areas.
The results of this study are consistent with prior research showing lower levels of self-reported physical function in individuals approaching a BMI of 30 kg/m2.34 Coakley et al34 found a significant dose-response relationship between increasing levels of BMI and lower levels of physical function. In women 45-71 years of age, function decreased by approximately 5.5% among the moderately overweight (BMI 28–29.9 kg/m2) compared to those of normal weight. Similar to findings of the current study, significantly lower levels of function were noted in women at higher levels of obesity. For example, those with a BMI > 30 kg/m2 experienced a 10% decrease in function and those with a BMI > 35 kg/m2 had 14% to 16% lower functioning compared to the normal weight reference group.34 The current findings indicate the normal weight and overweight groups were similar in function while the decrements in physical function occurred in those with obesity (ie, BMI > 30 kg/m2).
Other researchers have reported that physical function deteriorates at a higher BMI level (> 35 kg/m2) than that found in the current study.39 However, a difference in the way that functional impairment was defined is likely to account for the discrepancy. For example, Friedmann et al39 defined impairment in function as needing assistance with a functional activity. In the current study, impairment was defined as reporting a degree of difficulty with the functional task. As a result, the measure used by Friedmann et al39 was more likely to identify individuals at a later stage in the spectrum of disability compared to the self-report measure used in this study, which was likely to identify individuals at an earlier stage of decline.
On the performance-based measures, participants that were normal weight and overweight performed similarly; however, those classified as obese had poorer performance on each of the measures. Thus, these findings were consistent with self-reported limitations. In this sample of relatively younger, high-functioning females, only the normal weight and overweight groups achieved desirable gait speeds (> 1.2 m/s) based on previous studies in older adults.47 For each of the obese categories, the mean gait speed was < 1.2 m/s, which may have implications for these individuals to function successfully in the community. For example, in order to safely negotiate through a traffic intersection, an individual must be able to walk at a speed of 1.2 m/s.56 Furthermore, the mean gait speed of .97 m/s observed in participants in the obese class III category is not just indicative of impaired functioning, but in addition, individuals with usual gait speeds < 1.0 m/s are deemed higher risk for a number of adverse health events including nursing home admission, falls, and disability.47,57
The finding that gait speed is most impaired in individuals with class III obesity is supported in previous studies. In an analysis of the kinematic components of gait in adults with obesity, Spyropoulos and colleagues32 found that individuals with obesity walked much slower than those of normal weight (1.09 m/sec vs. 1.64 m/sec, p < .001). While these subjects had faster gait speeds than participants in the current study, the subjects were also younger in age (30 to 47 years).32 de Souza et al29 analyzed the gait of 34 obese individuals and found that subjects walked at a mean gait speed of .73 m/s, also placing them in a high risk category. In the latter study, participants were more similar in age to those in the current study (x = 47.2 years).29
The findings from the self-report and performance-based measures underscore the negative impact obesity has on physical function even in apparently healthy middle-aged women. As health professionals who specialize in the prescription of exercise to improve physical function and overall health status, physical therapists possess an integral role in primary, secondary, and tertiary prevention as it relates to this disease. To have an active role in the prevention and management of obesity, it is imperative that physical therapists are able to accurately identify clients/patients who are overweight and obese. More attention to a screening/examination process that includes assessment of body size, as well as potential contributing factors such as decreased physical activity level, may better position the profession to promote health behaviors in individuals who are obese.
Our study also demonstrated BMI had an impact on the relationship between self-report and performance-based measures of physical function. When BMI was added to the regression models examining the association between self-report and performance-based measures, BMI attenuated the relationship between the measures by approximately 50% indicating a significant influence of BMI on association between perception and performance.
The effect of BMI on the association between the measures may have been the result of several factors. For example, individuals with higher BMIs may exert more effort to perform functional tasks compared to those of normal weight. Studies have shown that individuals with obesity expend more energy during walking at preferred walking speeds compared to the non-obese.58,59 The increased energy cost associated with walking may result from a higher workload generated from a greater moment of inertia created by larger limbs, a reduction in mechanical efficiency during walking, or altered skeletal muscle efficiency in those with obesity.58,60–62 Furthermore, previous researchers have reported that individuals with obesity have an increased perception of exertion during walking.63,64 In the current study, individuals with obesity may have reported lower levels of physical functioning than they were capable of due to increased effort and perceived exertion required to complete the task.
In addition, perception of ability in those with higher BMIs may have also been influenced by discomfort, pain, or symptoms associated with comorbid health conditions. In the current study, participants with obesity reported a greater number of co-morbid health conditions and as a result, may have experienced more symptoms related to health conditions during walking. Previous studies have reported that obese individuals report more discomfort and musculoskeletal pain during functional walking tests compared to lean counterparts.63
Another explanation for a discrepancy between perception and ability could be related to physical activity level. Women in the obese groups were less active than those in the normal weight and overweight groups. Having been less physically active, participants with obesity may not have had an accurate perception of their abilities. This finding is supported in previous studies that have shown that perception of walking ability was related to physical activity level.42,65 Data from the Women's Health and Aging Study support that women who perceive they have difficulty walking are less active than those who perceive less difficulty.65 When implementing physical activity interventions, physical therapists should address the factors associated with perception of difficulty walking, such as perceived effort and associated symptoms that interfere with walking, to improve adherence and participation.
This study is not without limitations that could impact the application of the observed results. This study was cross-sectional in nature; thus, the direction of causality cannot be established. The study compared self-report and performance-based measures at one time point; thus, it cannot be determined if the association between the measures is maintained with repeated measures. This study did not take into account other lifestyle factors that may have influenced physical function such as current or former smoking, unhealthy diet, and alcohol use, all of which may have confounded the results. Future studies should consider controlling for lifestyle factors that have been shown to influence risk of functional limitation. Finally, individuals recruited for the study were volunteers and the characteristics of the sample (gender, age, physical activity level) were controlled to decrease the potential for confounding variables to influence physical function. Thus, our results may have been influenced by selection bias and the findings cannot be generalized to populations that do not match the characteristics of those in the study.
CONCLUSION
The results of this study expand on the current evidence linking obesity to limitations in physical function. In this study, individuals classified as normal weight and overweight were similar in physical function, while individuals with obesity had greater impairments in physical function. While those with severe obesity (eg, Class III) were most impaired, those with less severe obesity (eg, Class I and II) also demonstrated significant declines in physical function comparable to levels found in older adults.47 Body mass index appeared to have an influence on perception of functional abilities. The discrepancy between perception and ability may have resulted from greater perceived effort, the influence of symptoms such as pain, and/or lower physical activity level in the obese.63–66 These findings reinforce the need for physical therapists to identify individuals who are obese and promote behaviors to minimize the adverse health consequences, as well as the functional limitations related to obesity. Furthermore, when designing treatment programs for individuals with obesity, physical therapists should consider the functional benefits that can be achieved through exercise and/or weight loss, which may occur in conjunction with reduction of other health risks.
ACKNOWLEDGEMENTS
This research was supported by a student research grant to Dr. Hergenroeder from the University of Pittsburgh School of Education and the Research Development Fund from the School of Health and Rehabilitation Sciences.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119(7 Pt 2):655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 5.Mensah GA, Mokdad AH, Ford E, et al. Obesity, metabolic syndrome, and type 2 diabetes: emerging epidemics and their cardiovascular implications. Cardiol Clin. 2004;22(4):485–504. doi: 10.1016/j.ccl.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31(11 Suppl):S663–667. doi: 10.1097/00005768-199911001-00026. [DOI] [PubMed] [Google Scholar]
- 7.Hubert HB, Bloch DA, Fries JF. Risk factors for physical disability in an aging cohort: the NHANES I Epidemiologic Followup Study. J Rheumatol. 1993;20(3):480–488. [PubMed] [Google Scholar]
- 8.Houston DK, Ding J, Nicklas BJ, et al. The association between weight history and physical performance in the Health, Aging and Body Composition study. Int J Obes. 2007;31(11):1680–1687. doi: 10.1038/sj.ijo.0803652. [DOI] [PubMed] [Google Scholar]
- 9.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298(17):2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 10.Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9(2):147–153. [PubMed] [Google Scholar]
- 11.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization: Physical Status: The use and interpretation of anthropometry, Geneva, Switzerland: World Health Organization 1995. WHO Technical Report Series. [PubMed]
- 13.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3(1):73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 14.Blew RM, Sardinha LB, Milliken LA, et al. Assessing the Validity of Body Mass Index Standards in Early Postmenopausal Women. Obesity. 2002;10(8):799–808. doi: 10.1038/oby.2002.108. [DOI] [PubMed] [Google Scholar]
- 15.Evans EM, Rowe DA, Racette SB, Ross KM, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? Int J Obes. 2006;30(5):837–843. doi: 10.1038/sj.ijo.0803208. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate Physical Activity Intervention Strategies for Weight Loss and Prevention of Weight Regain for Adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 17.Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 18.Flechtner-Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G. Metabolic and Weight Loss Effects of Long-Term Dietary Intervention in Obese Patients: Four-Year Results. Obesity. 2000;8(5):399–402. doi: 10.1038/oby.2000.48. [DOI] [PubMed] [Google Scholar]
- 19.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 20.Tchernof A, Nolan A, Sites CK, et al. Weight loss reduces Creactive protein levels in obese postmenopausal women. Circulation. 2002;105(5):564–569. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 21.Fontaine KR, Barofsky I, Bartlett SJ, et al. Weight loss and health-related quality of life: results at 1-year follow-up. Eat Behav. 2004;5(1):85–88. doi: 10.1016/S1471-0153(03)00059-X. [DOI] [PubMed] [Google Scholar]
- 22.Jensen GL, Roy MA, Buchanan AE, et al. Weight loss intervention for obese older women: improvements in performance and function. Obes Res. 2004;12(11):1814–1820. doi: 10.1038/oby.2004.225. [DOI] [PubMed] [Google Scholar]
- 23.Kaukua J, Pekkarinen T, Sane T, et al. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification–a 2-y follow-up study. Int J Obes Relat Metab Disord. 2003;27(10):1233–1241. doi: 10.1038/sj.ijo.0802379. [DOI] [PubMed] [Google Scholar]
- 24.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. May 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 25.Villareal DT, Banks M, Sinacore DR, et al. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166(8):860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 26.American Physical Therapy Association Guide to physical therapist practice. 2nd ed. Phys Ther. 2001:81. [PubMed] [Google Scholar]
- 27.APTA Vision Sentence and Vision Statement for Physical Therapy 2020: http://www.apta.org/vision2020
- 28.Apovian CM, Frey CM, Wood GC, et al. Body mass index and physical function in older women. Obes Res. 2002;10(8):740–747. doi: 10.1038/oby.2002.101. [DOI] [PubMed] [Google Scholar]
- 29.de Souza SA, Faintuch J, Valezi AC, et al. Gait cinematic analysis in morbidly obese patients. Obes Surg. 2005;15(9):1238–1242. doi: 10.1381/096089205774512627. [DOI] [PubMed] [Google Scholar]
- 30.Ensrud KE, Nevitt MC, Yunis C, et al. Correlates of impaired function in older women. J Am Geriatr Soc. 1994;42(5):481–489. doi: 10.1111/j.1532-5415.1994.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 31.LaCroix AZ, Guralnik JM, Berkman LF, et al. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137(8):858–869. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 32.Spyropoulos P, Pisciotta JC, Pavlou KN, et al. Biomechanical gait analysis in obese men. Arch Phys Med Rehabil. 1991;72(13):1065–1070. [PubMed] [Google Scholar]
- 33.Sternfeld B, Ngo L, Satariano WA, et al. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. [see comment] Am J Epidemiol. 2002;156(2):110–121. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 34.Coakley EH, Kawachi I, Manson JE, et al. Lower levels of physical functioning are associated with higher body weight among middle-aged and older women. Int J Obes Relat Metab Disord. 1998;22(10):958–965. doi: 10.1038/sj.ijo.0800698. [DOI] [PubMed] [Google Scholar]
- 35.Galanos AN, Pieper CF, Cornoni-Huntley JC, et al. Nutrition and function: is there a relationship between body mass index and the functional capabilities of community-dwelling elderly? J Am Geriatr Soc. 1994;42(4):368–373. doi: 10.1111/j.1532-5415.1994.tb07483.x. [DOI] [PubMed] [Google Scholar]
- 36.Reuben DB, Valle LA, Hays RD, et al. Measuring physical function in community-dwelling older persons: a comparison of self-administered, interviewer-administered, and performance-based measures. J Am Geriatr Soc. 1995;43(1):17–23. doi: 10.1111/j.1532-5415.1995.tb06236.x. [DOI] [PubMed] [Google Scholar]
- 37.Davison KK, Ford ES, Cogswell ME, et al. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50(11):1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 38.Jensen GL. Obesity and functional decline: epidemiology and geriatric consequences. Clin Geriatr Med. 2005;21(4):677–687. doi: 10.1016/j.cger.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Friedmann JM, Elasy T, Jensen GL. The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc. 2001;49(4):398–403. doi: 10.1046/j.1532-5415.2001.49082.x. [DOI] [PubMed] [Google Scholar]
- 40.Jensen GL, Friedmann JM. Obesity is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc. 2002;50(5):918–923. doi: 10.1046/j.1532-5415.2002.50220.x. [DOI] [PubMed] [Google Scholar]
- 41.Lakdawalla DN, Bhattacharya J, Goldman DP. Are the young becoming more disabled? Health Affairs. 2004;23(1):168–176. doi: 10.1377/hlthaff.23.1.168. [DOI] [PubMed] [Google Scholar]
- 42.Brach JS, VanSwearingen JM, Newman AB, et al. Identifying early decline of physical function in community-dwelling older women: performance-based and self-report measures. Phys Ther. 2002;82(4):320–328. [PubMed] [Google Scholar]
- 43.Kuczmarski RJ, Carroll MD, Flegal KM, et al. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994) Obes Res. 1997;5(6):542–548. doi: 10.1002/j.1550-8528.1997.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 44.Sayers SP, Jette AM, Haley SM, et al. Validation of the Late-Life Function and Disability Instrument. J Am Geriatr Soc. 2004;52(9):1554–1559. doi: 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- 45.Hand C, Richardson J, Letts L, et al. Construct validity of the late life function and disability instrument for adults with chronic conditions. Disabil Rehabil. 2010;32(1):50–56. doi: 10.3109/09638280902998789. [DOI] [PubMed] [Google Scholar]
- 46.Dubuc N, Haley S, Ni P, et al. Function and disability in late life: comparison of the Late-Life Function and Disability Instrument to the Short-Form-36 and the London Handicap Scale. Disabil Rehabil. 2004;26(6):362–370. doi: 10.1080/09638280410001658667. [DOI] [PubMed] [Google Scholar]
- 47.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gaitspeed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 48.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88(6):733–746. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- 49.Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. Jan 1997;26(1):15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 50.Curb JD, Ceria-Ulep CD, Rodriguez BL, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54(5):737–742. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 51.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 52.King MB, Judge JO, Whipple R, et al. Reliability and responsiveness of two physical performance measures examined in the context of a functional training intervention. Phys Ther. 2000;80(1):8–16. [PubMed] [Google Scholar]
- 53.Rigler SK, Studenski S, Wallace D, et al. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16(4):420–428. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 54.Paffenbarger RS, Jr., Hyde RT, Wing AL, et al. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 55.Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57(11):M209–216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 56.Langlois JA, Keyl PM, Guralnik JM, et al. Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health. 1997;87(3):393–397. doi: 10.2105/ajph.87.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Eng J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster GD, Wadden TA, Kendrick ZV, et al. The energy cost of walking before and after significant weight loss. Med Sci Sports Exerc. 1995;27(6):888–894. [PubMed] [Google Scholar]
- 59.Lazzer S, Boirie Y, Bitar A, et al. Assessment of energy expenditure associated with physical activities in free-living obese and nonobese adolescents. Am J Clin Nutr. 2003;78(3):471–479. doi: 10.1093/ajcn/78.3.471. [DOI] [PubMed] [Google Scholar]
- 60.Ohrstrom M, Hedenbro J, Ekelund M. Energy expenditure during treadmill walking before and after vertical banded gastroplasty: a one-year follow-up study in 11 obese women. Eur J Surg. 2001;167(11):845–850. doi: 10.1080/11024150152717689. [DOI] [PubMed] [Google Scholar]
- 61.Saibene F, Minetti AE. Biomechanical and physiological aspects of legged locomotion in humans. Eur J Appl Physiol. 2003;88(4-5):297–316. doi: 10.1007/s00421-002-0654-9. [DOI] [PubMed] [Google Scholar]
- 62.Myers MJ, Steudel K. Effect of limb mass and its distribution on the energetic cost of running. J Exp Biol. 1985;116:363–373. doi: 10.1242/jeb.116.1.363. [DOI] [PubMed] [Google Scholar]
- 63.Hulens M, Vansant G, Claessens AL, et al. Predictors of 6-minute walk test results in lean, obese and morbidly obese women. Scand J Med Sci Sports. 2003;13(2):98–105. doi: 10.1034/j.1600-0838.2003.10273.x. [DOI] [PubMed] [Google Scholar]
- 64.Mattsson E, Larsson UE, Rossner S. Is walking for exercise too exhausting for obese women? Int J Obes Relat Metab Disord. 1997;21(5):380–386. doi: 10.1038/sj.ijo.0800417. [DOI] [PubMed] [Google Scholar]
- 65.Simonsick EM, Guralnik JM, Fried LP. Who walks? Factors associated with walking behavior in disabled older women with and without self-reported walking difficulty. J Am Geriatr Soc. 1999;47(6):672–680. doi: 10.1111/j.1532-5415.1999.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 66.Browning RC, Kram R. Energetic cost and preferred speed of walking in obese vs. normal weight women. Obes Res. 2005;13(5):891–899. doi: 10.1038/oby.2005.103. [DOI] [PubMed] [Google Scholar]