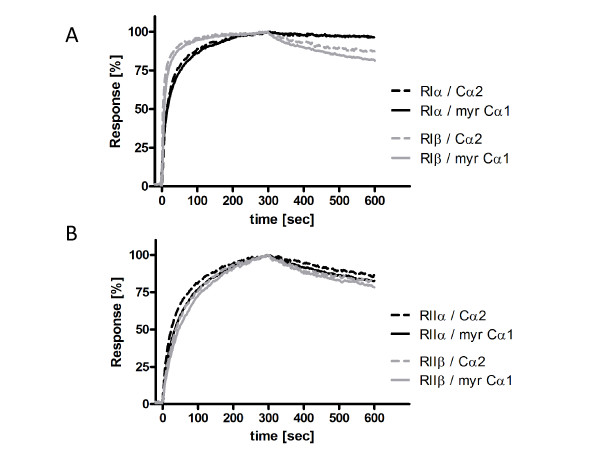
Figure 6.
The affinity of RI and RII subunits for Cα1 and Cα2 are comparable. Cα2 and myrCα1 were immobilized on a CM5 sensor chip each with 300 RUs of on a surface using amine coupling (see Methods). Then recombinant RIα and RIβ (panel A) as well as RIIα and RIIβ (panel B) were run simultaneously over each Cα surface monitoring the association and dissociation of each R subunit at a flow rate of 30 μl/min. Association and dissociation were recorded for 5 min each and the interaction was measured with R subunits in concentrations ranging from 0.25 nM to 128 nM and 0.5 nM to 256 nM for type I and type II enzymes (for details see results), respectively (table 1). All experiments were performed in 20 mM MOPS pH 7, 150 mM NaCl plus 0.005% (v/v) surfactant P20, 1 mM ATP, 5 mM MgCl2 and 50 μM EDTA. The panels show representative kinetics injecting the four R-isoforms on myrCα1 and Cα2 as indicated on the normalized plot using 64 nM of each R subunit.