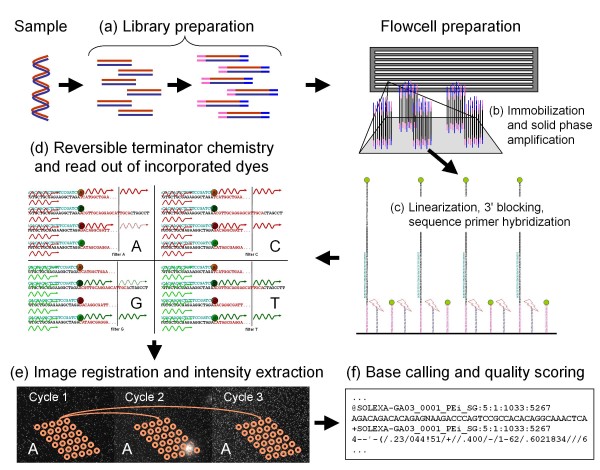
Figure 1.
Illumina sample preparation and sequencing. Illumina sequencing requires that a DNA sample (a) is converted into special sequencing libraries. This can be achieved by shearing DNA to a designated size and adding specific adapter sequences on both ends of the DNA molecules (b). These adapters allow molecules to be amplified and immobilized in one or more channels of an 8-channel flow cell (c). Immobilization and solid-phase amplification create randomly scattered clusters, consisting of a few thousand copies of the original molecule in very close proximity to each other. One of the DNA strands is removed to obtain single stranded, identically oriented copies, 3' ends of the DNA are blocked and a sequencing primer hybridized on the adapter sequences. Afterwards, the reversible terminator chemistry is performed (d). Here, four differently labeled nucleotides are provided and used for extension of the primers by DNA polymerases. The polymerase reaction terminates after the first base incorporation since the nucleotides used are not only labeled, but also 3'-blocked. After washing away free nucleotides, the nucleotides incorporated are readout by piece-wise imaging of the flow cell. Then, the terminator and fluorophore are removed and another incorporation cycle started. The four images are overlaid (registered) and light intensities extracted for each cluster and cycle using a cluster position template obtained from the first instrument cycles (e). Resulting intensity files serve as input for base calling, the conversion of intensity values into bases and quality scores (f).