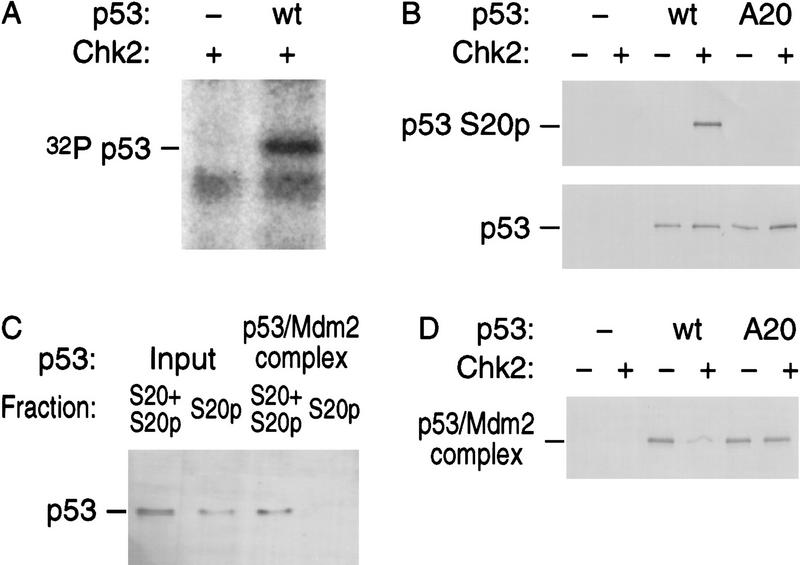
Figure 1.
Chk2/hCds1 phosphorylates p53 on Ser-20 in vitro and dissociates preformed p53/Mdm2 complexes. (A) Recombinant Chk2/hCds1 was incubated with [32P]ATP in the presence or absence of recombinant full-length p53. The reaction products were resolved by denaturing gel electrophoresis and visualized by autoradiography. (B) Full-length wild-type (wt) p53 or p53 with substitution of Ser-20 with Ala (A20) were incubated with Chk2/hCds1. Phosphorylation of p53 on Ser-20 (S20p) was monitored by immunoprecipitation of the reaction products with AbS20p, an antibody specific for p53 phosphorylated on Ser-20, followed by immunoblotting with antibody DO7, which recognizes p53 irrespective of its phosphorylation state. p53 protein levels were monitored by immunoblotting with DO7. (C) p53 phosphorylated by Chk2/hCds1 does not interact with Mdm2. Full-length p53 was incubated with Chk2/hCds1. One-half of the sample was stored on ice (input sample) and the other half was incubated with beads coated with GST–Mdm2(1–125). After washing the beads to remove unbound p53, the p53 protein bound to Mdm2 was eluted (Mdm2-bound sample). The fraction of p53 phosphorylated on Ser-20 in the input and Mdm2-bound samples was determined by comparing the amounts of p53 immunoprecipitated with antibodies AbS20p (p53 phosphorylated on Ser-20; S20p) and DO7 (p53 phosphorylated and nonphosphorylated on Ser-20; S20+S20p). (D) Chk2/hCds1 disrupts preformed p53/Mdm2 complexes. Complexes of GST–Mdm2 with wild-type p53 or p53A20 bound to glutathione beads were incubated with Chk2/hCds1. p53 that remained bound to Mdm2 after incubation of the beads with Chk2/hCds1 was resolved by denaturing gel electrophoresis and detected by immunoblotting with antibody DO7.